Assessing risk: The need for a new model
Although a number of breast cancer riskassessment models are available based on individual risk factors (TABLE 1), estimates based on combinations of factors are preferable. The Gail model,2 widely used to determine breast cancer risk, takes into account nongenetic (nulliparity, age at menarche) and genetic (family history) factors, as well as the number of previous breast biopsies. It assigns a smaller relative risk to women over age 50. A Web-based version, available at http://bcra.nci.nih.gov/brc, is useful for calculating a woman’s risk of developing invasive disease over the next 5 years, as well as over her remaining lifetime.
Limitations of the Gail model. Unfortunately, the data on which the model is based were collected in the late 1970s and early 1980s. Today, the greater ease of breast histopathologic assessment by fine-needle aspiration and outpatient core-needle biopsy has increased the rate of tissue sampling, creating confusion as to what constitutes a biopsy. Thus, the cutoff of 1.66% for high risk—the threshold adopted for the Breast Cancer Prevention Trial (BCPT)—loses some credibility.
Consider this example: A 50-year-old nulliparous Caucasian woman experienced menarche at age 11, has no first-degree relatives with a history of breast cancer, and has never had a breast biopsy. The Gail model would assign her a risk of developing breast cancer of 1.2% in the next 5 years and 10.8% over her lifetime. However, if the same patient had had 3 breast biopsies, her risk would rise to 1.8% in the next 5 years and 15.8% for her lifetime (placing her in the high-risk category), even if none of the biopsies revealed hyperplasia.
Biomarkers. Objective findings that are patient-specific but which correlate closely with breast cancer development are needed.
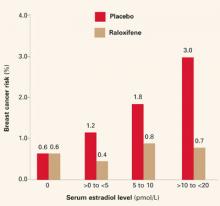
Biomarkers have been proposed; among them: ultrasensitive measurement of serum estradiol levels in postmenopausal women.3 In the Multiple Outcomes of Raloxifene Evaluation (MORE),4 the women who had the greatest reduction in breast cancer during treatment had the highest baseline serum estradiol levels (FIGURE 1)—although the baseline levels of all subjects were well within the postmenopausal range of 20 pmol/L or less.
TABLE 1
Breast cancer risk factors and their relative risks19
| RELATIVE RISK <2 | RELATIVE RISK 2–4 | RELATIVE RISK >4 |
|---|---|---|
|
|
|
FIGURE 1 Breast cancer risk in raloxifene users
Reprinted with permission from: Cummings SR, Duong T, Kenyon E, et al. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220. Copyright © 2002 American Medical Association. All rights reserved.
Chemoprevention: The rationale for tamoxifen
Data from preclinical animal and in vitro studies led to the use of tamoxifen, a selective estrogen receptor modulator (SERM), for primary prevention of breast cancer in healthy women. The drug was shown to inhibit mammary tumors in mice and rats and suppress hormone-dependent breast cancer cell lines in vitro.5
Clinical data from the Early Breast Cancer Trialists Collaborative Group also helped spur prevention trials with tamoxifen.6 Besides decreasing the risk of recurrent breast cancer, tamoxifen reduced the risk of contralateral, new-onset breast cancer by 47% after 5 years of adjuvant treatment (P = .00001) and by 26% after 2 years of treatment (P = .004). This, along with tamoxifen’s favorable effects on skeletal remodeling and lipid levels, led to a series of chemopreventive trials in the United States and Europe (TABLE 2).
TABLE 2
Results of tamoxifen trials
| BREAST CANCER PREVENTION TRIAL7 | ROYAL MARSDEN10 | ITALIAN11 | |
|---|---|---|---|
| Number of participants | 13,388 | 2,471 | 5,408 |
| Age ≤50 | 40% | 62% | 36% |
| One first-degree relative with breast cancer | 55% | 55% | 18% |
| >2 first-degree relatives with breast cancer | 13% | 17% | 2.5% |
| HRT users | 0% | 42% | 8% |
| Woman-years of follow-up | 46,858 | 12,355 | 20,731 |
| Cancer incidence/1,000 | |||
| Placebo | 6.8 | 5.0 | 2.3 |
| Tamoxifen | 3.4 | 4.7 | 2.1 |
Breast Cancer Prevention Trial: Tamoxifen reduces cancer incidence
In 1992, the National Surgical Adjuvant Breast and Bowel Project launched a prevention trial using tamoxifen: the Breast Cancer Prevention Trial.7 A total of 13,388 women aged 35 or older and at high risk for breast cancer were enrolled at numerous sites throughout the United States and Canada.
The Gail model was utilized to determine which women had sufficient risk—that is, a risk of developing invasive breast cancer within the next 5 years of 1.66% or greater—to be included in the trial.2 Subjects were randomly assigned to receive placebo or tamoxifen (20 mg/d) for 5 years.
The trial was terminated in April 1998, 14 months before its planned completion, due to the striking reduction in new-onset breast cancer in the tamoxifen group. The data safety monitoring board felt it would be unethical to allow one half of the participants, who were deemed to be at high risk, to continue taking placebo in light of the dramatic reduction in both invasive and noninvasive breast cancer with tamoxifen use.