The author reports no financial relationships relevant to this article.
- What clinical problem is this device or drug designed to address?
- Is the problem important clinically for the well-being of my patients?
- What do I know about the basic science and physiology of the problem?
- Are there data supporting improvement in clinical outcomes, or do the studies look only at surrogate endpoints?
- Has the drug or device been studied in gynecologic patients? Is it approved for use in this population?
- Is there evidence that the new technology is more effective, safer, or less expensive than products I use now?
The process of evaluation is critical for us—as advocates for individual patients and as stewards of the diminishing resources available to treat patients in the United States.
With these questions in mind, let’s look at a surgical problem for which there are many new and potentially exciting products being promoted to gynecologic surgeons: the need to achieve hemostasis.
Bleeding is a serious clinical problem during surgery—one that can have a major impact on the well-being of the patient. We generally use sutures and electrosurgical instruments—both bipolar and monopolar—to control major vessels. Topical hemostasis agents may be useful, however, in areas where generalized oozing is present, or where the application of energy may endanger vital structures.
Many products are available for use in these situations. Some are tried and true; others, new or unapproved for use in gynecologic surgery. Your assessment and selection of the optimal product should take into account 1) the cause of bleeding and 2) the mechanism of action of the product.
Surgical bleeding has one of two causes
Although the science of coagulation is complex, bleeding occurs, from a surgical perspective, because of either of two problems:
- failure to control significant arterial and venous sources
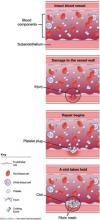
- failure of normal clotting functions, such as vasoconstriction; platelet activation and plugging ( FIGURE ); and activation of the coagulation cascade.
No topical or systemic agent adequately controls Problem #1. To address Problem #2, we have several choices to augment hemostasis, including thermal, chemical, and mechanical means. In addition, newer agents deliver additional coagulation-enhancing products such as platelets and thrombin to the operative site to supplement the patient’s natural clotting process.
FIGURE How a clot forms
Tried and true technologies
Because of long-standing experience with porcine gelatin, oxidized regenerated cellulose (ORC), and microfibrillar collagen, the Food and Drug Administration recategorized them in 2002 as Class-II devices with a clear safety profile.
Porcine gelatin
This class of products (Gelfoam, Surgifoam, Spongistan) has been around since the 1940s. The products have no intrinsic hemostatic action. They absorb 45 times their weight in blood and provide a scaff old on which platelets come into close contact, initiating the release of intrinsic and extrinsic clotting mechanisms.
Oxidized regenerated cellulose
This class (Surgicel, Oxycel) has also been around since the 1940s. The agents have acidic properties, due to their low pH level, and achieve hemostasis via denaturation of blood proteins, mechanical activation of the clotting cascade, and local vasoconstriction.
Because of its low pH, ORC is bactericidal against many common pathogens of the reproductive tract. A few studies have explored laparoscopic application of ORC to achieve hemostasis at sites of uterine perforation and for tubal bleeding secondary to sterilization.1,2 Successful hemostasis of moderate bleeding was achieved without the need for suture or conversion to laparotomy in all cases without brisk arterial bleeding.
Another ORC product (Interceed) is often used in gynecologic surgery as a barrier to adhesion formation. Its degree of oxidation, weave, and pore size differ from those of Surgicel. It requires an absolutely dry operative field to prevent adhesions.
Microfibrillar collagen
Substances derived from bovine collagen were marketed in the 1970s and 1980s. Collagen provides binding sites for platelets, which degranulate, releasing coagulation factors and initiating the clotting cascade.