Innovation over 5 decades has added options to the medicine chest of contraceptives that we can offer to our patients. The US Food and Drug Administration recently approved Natazia (Bayer HealthCare), a novel four-phase, estrogen step-down, progestin step-up regimen that contains a new estrogen, estradiol valerate, and the novel progestin, dienogest. Here is how this OC is constituted and how it works.
Why estradiol valerate?
Almost all oral estrogen-progestin contraceptives contain the potent estrogen ethinyl estradiol. The ethinyl group at C17 prevents conversion of this highly active estrogen to the weak estrogen, estrone, thereby extending its estrogenic bioactivity.
High estrogenic activity, and limited degradation of oral ethinyl estradiol, may account for its pronounced effects on the production of proteins in the liver, including 1) an increase in thrombotic proteins and 2) a decrease in some antithrombotic proteins. It’s been speculated that using the natural estrogen, estradiol, in an oral estrogen-progestin contraceptive would reduce the magnitude of the liver protein changes observed with ethinyl estradiol. This, in turn, would (again, this is speculation) reduce the risk of deep-vein thrombosis, which has been observed among women who use contraceptives that contain ethinyl estradiol.1,2
Early attempts to formulate a monophasic oral estrogen-progestin contraceptive that contains estradiol and estriol did yield OCs that inhibited ovulation, but those products were associated with a high rate of irregular bleeding patterns, including amenorrhea and spotting.3
Now, the newly approved estrogen-progestin contraceptive, Natazia, which contains estradiol valerate (FIGURE 1) appears to over-come those unwanted side effects.
When estradiol valerate is taken orally, the intestines absorb almost all of it. The valerate group is cleaved within the intestines or in the first pass through the liver—thus converting the compound to estradiol and valeric acid. A woman who takes Natazia has a circulating level of estradiol of approximately 50 pg/mL and a higher level of estrone (250 pg/mL); this difference reflects the robust conversion and rapid degradation of orally administered estradiol to estrone.
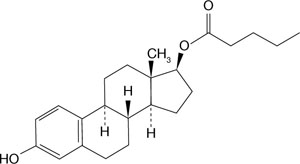
FIGURE 1 The structure of estradiol valerate
With absorption, estradiol valerate is metabolized to estradiol. Estradiol is metabolized to estrone and estrone sulfate.
Dienogest: Novel progestin
This new 19-nortestosterone derivative has a unique C-17 cyanomethyl group instead of the usual C-17 ethinyl grouping (FIGURE 2). Dienogest binds most avidly to the progesterone receptor; it does not bind to estrogen, glucocorticoid, or miner-alocorticoid receptors. It binds weakly to the androgen receptor, and reportedly has antiandrogenic activity.
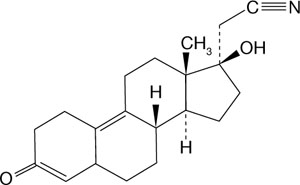
FIGURE 2 The structure of dienogest
This new 19-nortestosterone derivative has a unique C-17 cyanomethyl group, not the usual C-17 ethinyl group.
When dienogest is taken orally, more than 90% of the dose is absorbed; half-life is approximately 11 hours. It appears to have unparalleled potency among oral progestins when tested in estrogen-primed rabbit endometrium (McPhail test).4
Dienogest is metabolized by the cytochrome P-450 CYP3A4 system. Concomitant administration of dienogest and CYP3A4 inducers— barbiturates, carbamazepine, phenytoin, primidone, rifampicin—may decrease the time needed to clear dienogest. Concurrent administration of dienogest and CYP3A4 inhibitors— antidepressants, azole antifungals, cimetidine, grapefruit juice, diltiazem, macrolides, verapamil—may increase the circulating concentration of dienogest.
Estrogen step-down, progestin step-up
Natazia is formulated as a four-phase estrogen step-down (from 3 mg to 1 mg), progestin step-up (from 2 mg to 3 mg) regimen (TABLE). A 28-day cycle has 26 pills containing hormones and two inert pills. Underlying this formulation are the theoretical concepts that:
- estrogen step-down ensures estrogen dominance in the first part of the cycle, thus stimulating endometrial proliferation and increasing endometrial production of progesterone receptors, which, in turn, sensitizes endometrium to the action of progestin
- progestin step-up ensures the stability of endometrial stroma and prevents endometrial hyperplasia.
Profile of four-phase Natazia
| Day of cycle | Pill color | Pill count | Estradiol valerate content | Dienogest content |
|---|---|---|---|---|
| 1, 2 | Dark yellow | 2 | 3 mg | None |
| 3–7 | Medium red | 5 | 2 mg | 2 mg |
| 8–24 | Light yellow | 17 | 2 mg | 3 mg |
| 25, 26 | Dark red | 2 | 1 mg | None |
| 27, 28 | White | 2 | None | None |
Comparative study. The clinical effect of Natazia was explored in a randomized controlled trial in which it was compared with a monophasic estrogen–progestin combination of 20 μg ethinyl estradiol plus 0.1 mg levonorgestrel (EE-LNG) in a 21-7 cycle (a formulation found in the brand-name OCs Aviane, Alesse, and Lutera).5 The findings of that trial?
- Both formulations were very effective: No pregnancies in women who took Natazia, one pregnancy in women who took EE-LNG
- In the first 90 days of the trial, women taking Natazia reported fewer days of bleeding or spotting than women taking EE-LNG (17 days and 22 days, respectively [P < .0001])
- Absence of withdrawal bleeding was observed more often in women taking Natazia than in women taking EE-LNG: With Natazia, about 19% of cycles were associated with an absence of withdrawal bleeding; with EE-LNG, 8% of cycles
- Approximately 80% of subjects in each of the two study groups were satisfied with the OC they were given.


