The author reports that he serves on the advisory boards of Amgen, Boehringer Ingelheim, Depomed, Eli Lilly, and Novo Nordisk. He is a speaker for the Alliance for Bone Health, Eli Lilly, and Warner Chilcott.
Some hormonal contraceptives may affect bone density
Berenson AB, Rahman M, Breitkopf CR, Bi LX. Effects of depot medroxyprogesterone acetate and 20-microgram oral contraceptives on bone mineral density. Obstet Gynecol. 2008;112:788–799.
American College of Obstetricians and Gynecologists. ACOG Committee Opinion. No. 415. September 2008. Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2008;112:727–730.
A woman’s contraceptive choice may affect her bone mineral density (BMD)—particularly if she chooses depot medroxyprogesterone acetate (DMPA) or a very-low-dose oral contraceptive (OC) as her method.
In the case of DMPA, studies have shown that its use for 2 years significantly impairs BMD at the hip and spine, regardless of the patient’s age, although BMD usually rebounds after discontinuation of the drug.1
In the case of very-low-dose OCs, there is evidence that young women who use a pill that contains only 20 μg of ethinyl estradiol have a lower increase in BMD than do women the same age who do not use hormonal contraception. (OCs that contain a higher dosage of ethinyl estradiol have not been shown to hamper BMD.) A study by Polatti and colleagues reported a 7.8% increase in BMD over 5 years among women 19 to 22 years old who did not use OCs, compared with no change in BMD among women who used OCs containing 20 μg of ethinyl estradiol.2
DMPA, BMD, and the FDA
The deleterious effect of DMPA on BMD is particularly relevant in perimenopausal women, who have already begun to experience the age-related decline in BMD that starts around the age of 30. The effect is also troubling in adolescents, who normally experience a large accretion of bone during the teen and early adult years.
In 2004, the US Food and Drug Administration (FDA) added a boxed warning to DMPA labeling advising women to limit their use of the drug to 2 years. Since that time, other studies have found that BMD increases to a greater degree among past users of DMPA than among never users—suggesting that DMPA-related bone loss is reversible.
ACOG: DMPA can be used longer than 2 years in some
In September 2008, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion acknowledging the association between DMPA and BMD loss. The committee pointed out, however, that “current evidence suggests that partial or full recovery of BMD occurs at the spine and at least partial recovery occurs at the hip after discontinuation of DMPA” ( FIGURE ).
The ACOG opinion also noted that, “given the efficacy of DMPA, particularly for populations such as adolescents, for whom contraceptive adherence can be challenging, or for those who feel they could not comply with a daily contraceptive method or a method that must be used with each act of intercourse, the possible adverse effects of DMPA must be balanced against the significant personal and public health impact of unintended pregnancy.”
The committee recommended that, despite concerns about bone loss, practitioners should not hesitate to prescribe DMPA. Nor should they limit its use to 2 consecutive years or perform BMD monitoring solely in response to DMPA use. “Any observed short-term loss in BMD associated with DMPA use may be recovered and is unlikely to place a woman at risk of fracture during use or in later years,” the committee opinion noted.
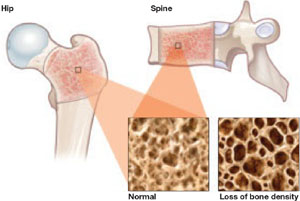
FIGURE DMPA-related bone loss is largely reversible
Depot medroxyprogesterone acetate (DMPA) is associated with a loss of bone mineral density at the hip and spine. Once the drug is discontinued, however, bone density appears to recover at least partially at both sites.
Study explores bone gains after DMPA
Berenson and associates measured BMD every 6 months for as long as 3 years in 703 white, African-American, and Hispanic women who used an OC, DMPA, or nonhormonal contraception. They also measured BMD for up to 2 additional years in 68 women who discontinued DMPA. They found no differences between races—although they did find the expected DMPA-associated bone loss.

