Brubaker L, Richter HE, Visco A, et al: Pelvic Floor Disorders Network. Refractory idiopathic urge urinary incontinence and botulinum A injection. J Urol. 2008;180:217–222.
Women who fail medical management of urge incontinence have few other options. This multicenter, randomized, double-blind, placebo-controlled trial suggests that there may one day be an effective alternative. Investigators examined the safety and efficacy of botulinum toxin type A (Botox) for the treatment of refractory idiopathic urge incontinence in 43 women—28 of them randomized to injection of 200 U of Botox and 15 to placebo.
Sixty percent of subjects in the Botox arm reported an improvement in symptoms, with a median response of 373 days, compared with 62 days in the placebo arm (P<.0001). Moreover, in the Botox arm, women perceived greater improvement in symptom control and a decrease in the number of self-reported incontinence episodes, compared with the placebo group (P<.0001).
However, 12 of 28 patients (43%) who received Botox developed elevated postvoid residuals (i.e., retention of more than 200 mL of urine), and enrollment was halted for this reason. Median time to initiate intermittent self-catheterization was 30 days, and intermittent self-catheterization lasted a median of 60 days. Nine of the 12 subjects who required self-catheterization developed a urinary tract infection.
Details of the trial
This study was conducted by the Pelvic Floor Disorders Network and sponsored by the National Institute of Child Health and Human Development. To be eligible for the trial, women had to have been diagnosed with refractory urge incontinence, which was defined as persistent symptoms after failing at least two first-line therapies such as anticholinergic medications and behavioral therapy. Also required was documented evidence of detrusor overactivity on urodynamic studies or at least six episodes of urge-related incontinence in a 3-day bladder diary. Investigators determined that a sample size of 210 subjects was needed to test a 50% efficacy rate for Botox, compared with 30% for placebo, at 6 months.
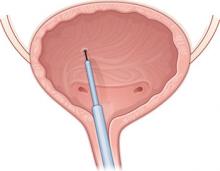
Baseline characteristics were similar between groups. A blinded physician used a cystoscope to inject the agents into the detrusor muscle over the posterior bladder wall ( FIGURE 2 ). Subjects also received an antibiotic before the procedure and for 3 additional days.
The primary outcome measure was treatment failure, defined as the return of symptoms measured at least 2 months after Botox injection or any change in medical therapy.
Enrollment was halted after interval analysis revealed a significantly higher rate of voiding dysfunction in the Botox arm, necessitating intermittent self-catheterization and associated urinary tract infections.
FIGURE 2 Intradetrusor Botox injection
In the trial by Brubaker and colleagues, 200 U of Botox was injected into the detrusor muscle on the posterior bladder wall to treat refractory urge incontinence in 28 women—60% of whom reported improvement.
Is a postvoid residual clinically significant?
Some experts questioned the clinical significance of the elevated postvoid residual reported in this trial, arguing that it was temporary and that many patients were asymptomatic.
Further study is needed to determine the optimal dosing of Botox and a management strategy for postprocedure voiding dysfunction.
When one of your patients considers cystoscopic intradetrusor Botox therapy for refractory urge incontinence, you should:
- counsel her extensively that its use here is not FDA-approved
- caution her that she may need to perform intermittent self-catheterization after injection
- advise her that the procedure and the medication are likely not covered by her health insurance and will be out-of-pocket expenses.
Much-needed and much-anticipated clinical trials are under way with the aim of obtaining FDA approval of Botox for urge incontinence.
At the Cleveland Clinic, we currently offer Botox for refractory urge incontinence.
InterStim therapy may become the gold standard for refractory overactive bladder syndrome
van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol. 2007;178:2029–2034.
Sacral nerve stimulation has been approved for use in patients with refractory voiding dysfunction since 1997. This prospective, worldwide, follow-up study sought to determine the long-term efficacy and safety of sacral neuromodulation for the treatment of refractory urgency, frequency, urge incontinence, and nonobstructive urinary retention.
After 5 years of follow-up, 68% of subjects who had urge incontinence, 56% who had urgency and frequency, and 71% who had nonobstructive urinary retention reported a degree of improvement of 50% or more in their symptoms (relative to baseline). The mean number of urge incontinence episodes decreased from 9.6 to 3.9, and mean voids per day decreased from 19.3 to 14.8 at 5 years (P<.001).