DANA POINT, CALIF. – Nonablative fractional photothermolysis technology is a well-suited model for over-the-counter product development, especially within the 1430 nm to 1450 nm range, Dr. Brian S. Biesman said at a meeting sponsored by SkinCare Physicians and Northwestern University.
"I want to dispel the myth that there’s nothing in the home-use realm that works," said Dr. Biesman, director of the Nashville (Tenn.) Center for Laser and Facial Surgery. "There is a lot of money in the investment community tied up in the home-use realm, and there are some real significant devices in this area."
Considerations for adoption of intense pulsed light and laser devices for home use should involve "the exact same standards that we apply to the devices that we use in the office," Dr. Biesman said. These include the safety of core technology, in both use and misuse settings: tolerability, predictable efficacy, ease of use, affordable cost, robust premarket evidence, and alignment between claims and reality.
Three nonablative fractional laser options exist for home-based treatment of photodamaged skin: the 1435-nm Palomar PaloVia, the 1410-nm Solta RéAura (not yet FDA cleared), and the 1410-nm Tria Beauty SRL, which is also pending FDA clearance.
Dr. Biesman discussed the Tria SRL, a 1440-nm fractional nonablative laser device that can deliver energy up to 260 microns in depth with an adjustable energy range of 5-12 millijoules/pulse. "At first, given the parameters within which this device operated, I didn’t expect it to be clinically useful," Dr. Biesman noted.
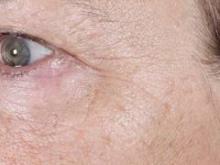
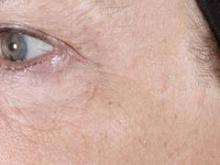
In a safety, efficacy, and tolerability study sponsored by Tria, 90 patients aged 32-70 years old underwent treatment for dyschromia, periorbital wrinkles, and textural irregularities on the face. Of the 90 patients, 87 were women, 87 were white, and 62% had Fitzpatrick skin types II or III.
Patients underwent full face treatment 5 days/week for 12 weeks. They were then followed at 1 day, 2 weeks, 4 weeks, 8 weeks, and 12 weeks after the final treatment. Standard and polarized photos were taken on a VISIA CR system by Canfield Scientific, at baseline, every 2 weeks during treatment, and at each follow-up visit. Blinded investigators used a validated nine-point scale to evaluate each indication.
Dr. Biesman, who was not an investigator in the study, reported that investigator scoring showed statistically significant and clinically meaningful improvements in texture, periorbital wrinkles, and discoloration at 4 weeks and 12 weeks post treatment (all with a P value of less than.001). Common side effects included erythema, stinging/prickling sensations, and warm sensations. All side effects were reported to be mild and self-resolving, and no serious adverse events were reported.
Self-reported patient satisfaction ranged from 80%-90%, "which are similar numbers if you look at the subject satisfaction for the office-based nonablative devices," Dr. Biesman said.
Dr. Biesman advises clinicians to think of home-use laser devices for the treatment of photoaging, acne, and hair reduction "as prescriptives, much as we would retinoids. They’re not going to replace what we do in the office," he said. "But if someone has made a substantial investment for an office-based treatment plan, why not recommend something they can use at home that will help them maintain that outcome?"
In his opinion, nonablative resurfacing is "the next area of great opportunity" in home devices. "But I think the area of greatest opportunity is using these nonablative devices with other over-the-counter or prescriptive topical agents for laser-enhanced drug delivery," he noted.
"Using this approach, I believe we can accomplish some unique and very interesting objectives. This is an area that is only just beginning to be explored, but which holds tremendous potential. I look forward to the future of these devices as stand-alone treatments and to enhance drug delivery to facilitate reaching challenging therapeutic and aesthetic endpoints," he added.
Dr. Biesman disclosed that he is a consultant for and has received travel funds from Tria Beauty.
*Correction, 9/26/2013: An earlier version of this story included incorrect image order and captions.