Cases in Menopause

Your postmenopausal patient reports a history of migraine
Would a trial of hormone therapy increase her risk of stroke?
Andrew M. Kaunitz, MD
Dr. JoAnn V. Pinkerton and Dr. James A. Simon provided peer review and comments for Dr. Kaunitz's case study.
Andrew M. Kaunitz, MD | |
JoAnn V. Pinkerton, MD | |
James A. Simon, MD |
Disclosures
Dr. Kaunitz reports that his institution receives grant or research support from Bayer, Endoceutics, Noven, and Teva, and that he is a consultant to Actavis, Bayer, DepoMed, and Teva.
Dr. Pinkerton reports that her institution receives consulting fees from DepoMed, Noven, NovoNordisk, Pfizer, and Shionogi; grant or research support from DepoMed, Bionova, and Endoceutics; and travel funds from DepoMed, Noven, NovoNordisk, Pfizer, and Shionogi.
Dr. Simon reports being a consultant to or on the advisory boards of Abbott Laboratories, Amgen, Ascend Therapeutics, DepoMed, Lelo, MD Therapeutics, Meda Pharmaceuticals, Merck, Noven, NovoNordisk, Novogyne, Pfizer, Shionogi, Shippan Point Advisors LLC, Sprout Pharmaceuticals, Teva, Warner Chilcott, and Watson. He also reports receiving (currently or in the past year) grant/research support from NovoNordisk, Novogyne, Palatin Technologies, Teva, and Warner Chilcott. He reports serving on the speakers bureaus of Noven, NovoNordisk, Teva, and Warner Chilcott. Dr. Simon was the Chief Medical Officer for Sprout Pharmaceuticals until April 1, 2013.

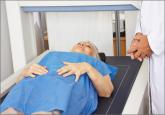
At age 61, because of her maternal history of osteoporosis and her own low BMI, the patient undergoes BMD assessment with dual x-ray absorptiometry (DXA). In average-risk women, NAMS recommends that BMD assessment be performed at age 65.31 The results of BMD assessment are normal.
After further discussion, the patient agrees to an even lower dose of estradiol, switching to an 0.025-mg patch, with progesterone (100 mg nightly) administered for 2 weeks in every 3-month interval. She reports no VMS or vaginal bleeding on this lower-dose HT regimen.
After 12 months on this new regimen, the patient undergoes vaginal sonography, revealing an endometrial thickness of 3 mm. She continues this regimen, including annual vaginal ultrasound assessment of the endometrium, without problems until her well-woman visit at age 65.
At that visit, I explain that discontinuation of HT is unlikely to trigger recurred VMS but may cause her to lose BMD rapidly for several years, and may also result in unpleasant symptoms from vulvovaginal atrophy including sexual discomfort. She decides to switch to an 0.014-mg estradiol patch without progesterone, and to undergo ultrasound assessment of her endometrium every 1 to 2 years.
Do you have a troubling case in menopause?
Suggest it to our expert panel. They may address your management dilemma in a future issue!
Email us at obg@frontlinemedcom.com
BOTTOM LINE: INDIVIDUALIZE THE DURATION OF HT
Although published data on extended use of HT are few, many clinicians caring for menopausal women are asked to make a recommendation. Because extended use of estrogen-progestin HT increases the risk of breast cancer, estrogen-only HT has a more favorable benefit-risk ratio. If a patient uses estrogen-progestin HT for an extended duration, periodic discussions about the elevated risk of breast cancer are appropriate.
JoAnn V. Pinkerton, MD:
The risk of breast cancer associated with extended use of estrogen-progestin HT likely is reduced if lower doses are given. Overall, however, the risk appears to be both dose- and duration-dependent.
We lack randomized trial data on CHD and other risks in women who begin HT at the time of menopause and continue it for decades. In older women who use HT for an extended duration, transdermal estrogen may be safer in regard to the risk of VTE and stroke.
As the systemic estrogen dose is lowered, it is possible to reduce the dose of the progestin (the sole function of which is to protect the endometrium from estrogen stimulation). Intermittent dosing can be used, although we lack long-term safety data, and periodic endometrial evaluation should be considered.
Remember also that, with intermittent or daily dosing of a progestin, you are relying on the patient to take this medication to protect the endometrium.
Extended use of low-dose vaginal estrogen HT may be necessary to treat symptoms of vulvovaginal atrophy, which tend to worsen over time. Administration of a progestin is not currently recommended with use of vaginal estrogen, but long-term use may increase the risk of endometrial stimulation.
Read other CASES IN MENOPAUSE
Your postmenopausal patient reports a history of migraine James A. Simon, MD (October 2013)
Your menopausal patient's breast biopsy reveals atypical hyperplasia JoAnn V. Pinkerton, MD (May 2013)
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com

Would a trial of hormone therapy increase her risk of stroke?
How do you now manage her menopausal symptoms, including bothersome hot flashes?
Dr. Kaunitz describes how he counsels patients about hormone therapy
Key findings and guidance from the past year on hot flushes, early menopause, and the hormone therapy–venous thromboembolism link. Plus, NAMS...
For postmenopausal women with newly diagnosed osteoporosis, assessing the risk of breast cancer prior to prescribing a bone medicine could...
The new data on hormone therapy (HT) suggest that we still have much to learn about its benefits and risks. We also are reaching an understanding...

YES. Women using estradiol had a lower risk of incident venous thromboembolism than women using conjugated equine estrogens (CEE), according to...

START individualizing therapy to optimize health and quality of life
