Commentary

In the previous edition of Master Class in Gynecologic Surgery, I described the controversy concerning electromechanical power morcellation freely in the abdominopelvic cavity. I also discussed the risks, as noted in the literature, of spreading an unsuspected leiomyosarcoma, and thus, up-staging the disease and lowering both the length of the disease-free state and the overall survival. I also talked about the lack of an adequate diagnostic study to definitively separate a leiomyoma from a leiomyosarcoma, discussed the at-risk population group, and also noted the benefits of minimally invasive gynecologic surgery. At that time, I stated I was against a ban on the morcellator, and that I recommended that physicians provide proper informed consent and, when possible, consider other treatment options, especially in the at-risk population. These included continued use of minimally invasive gynecologic surgery utilizing a specimen bag for electromechanical power morcellation.
Following that publication, the Food and Drug Administration released a statement regarding electromechanical power morcellation on April 17, 2014. While "discouraging" the use of electromechanical power morcellation, they wisely did not call for a moratorium. Again, the FDA recommended that patients be properly informed as to risk and that alternative therapies be discussed, which included the use of power morcellation in a bag.
While the FDA did not ban electromechanical power morcellation, the phrase "discourages the use" sent widespread ripples throughout our specialty. Within a week, Ethicon Endo-Surgery pulled its morcellator off the market worldwide. My hospital system – Advocate Health Care – as well as virtually every hospital in Boston placed a moratorium on electromechanical power morcellation. Dr. Jim Tsaltas, president of the Australian Gynecological Endoscopy & Surgery Society (AGES), was contacted by his country’s FDA equivalent to discuss the use of power morcellation freely in the abdominopelvic cavity.
Since the letter from the FDA, position papers have come from both the world’s largest society focused on minimally invasive surgery, the AAGL, and the American College of Obstetricians and Gynecologists (ACOG). Both of the society’s statements agree that proper informed consent is imperative and that alternate treatment be considered, especially in the at-risk population. Although both papers discuss the use of electromechanical power morcellation in the confines of a bag, the ACOG statement accurately notes that there is very little data regarding morcellation in a bag placed in the abdominopelvic cavity.
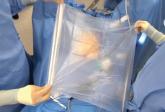
Experience with electromechanical power morcellation in a bag placed in the abdominopelvic cavity is now quickly developing. To quote my coauthor, Dr. Ceana Nezhat, "My goal with this Master Class is to provide a forum for discussion and encourage gynecologic surgeons to continue to practice minimally invasive surgery for the benefit of their patients. Despite the current limitation on unprotected, intraperitoneal electromechanical morcellation, the hope is that surgeons will not revert back to laparotomy, but will continue to learn and find innovative ways to provide the least invasive surgical techniques or refer to centers that can provide these services to women."
In this edition of Master Class in Gynecologic Surgery, I am featuring techniques of electromechanical power morcellation in a specimen bag by early adapters and innovators, and other techniques by Dr. Tony Shibley, Dr. Bernard Taylor, and Dr. Ceana H. Nezhat.
Dr. Shibley has been in full-time practice with Ob.Gyn. Specialists, Fairview Health Services in the Minneapolis area (Edina), for the past 20 years. He has a focus on single-site surgery and has been involved in minimally invasive surgical education both nationally and internationally, as well as in medical device development.
Dr. Taylor is a urogynecologist who is a female pelvic medicine and reconstructive surgeon practicing at the Carolinas Medical Center–Advanced Surgical Specialties for Women in Charlotte, N.C. He lectures nationally and internationally on minimally invasive gynecologic surgery.
Dr. Nezhat is the current president of the AAGL, adjunct professor of obstetrics and gynecology at Emory University and program director of minimally invasive surgery at Northside Hospital, both in Atlanta, and adjunct clinical professor of obstetrics and gynecology at Stanford (Calif.) University.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy , and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column. Dr. Miller disclosed that he is a consultant to Ethicon Endo-Surgery.
Videos of our experts’ individual techniques of electromechanical power morcellation within the confines of a bag, as well as that of Dr. Douglas Brown, director of the Center for Minimally Invasive Gynecologic Surgery at Massachusetts General Hospital, Boston, can be viewed at the SurgeryU website. Also at SurgeryU is a video of the electromechanical power morcellation technique in a bag that my partner, Dr. Aarathi Cholkeri-Singh, and I utilize. We use a 3,100-cc ripstop nylon specimen bag from Espiner Medical and the 5 x 150 mm, extra-long, shielded-bladed balloon-tipped trocar from Applied Medical. Go to SurgeryU to view videos of the procedures.

