Arnold Advincula's Surgical Techniques
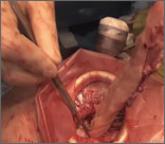

Can leiomyosarcoma be detected preoperatively?
OBG Management: How can we improve preoperative detection of uterine malignancy, particularly leiomyosarcoma?
Dr. Fader: For starters, we can improve detection of uterine cancers by simply looking for them. Almost all epithelial uterine cancers will be detectable by endometrial sampling. A comprehensive history and physical and uterine imaging also may be helpful. And although they are more difficult to detect than epithelial uterine cancers, it is a myth that sarcomas cannot be diagnosed preoperatively. Investigators from Columbia University retrospectively evaluated the ability to preoperatively detect epithelial uterine cancers and uterine sarcomas on final pathology. In 72 women who were ultimately diagnosed with a sarcoma, preoperative endometrial sampling suggested an invasive tumor in 86% and predicted the correct histology in 64%. In fact, the rate of detection of invasive cancer by preoperative sampling was not statistically different among sarcomas than it was among epithelial uterine cancers, although there was less of a correlation with appropriate histology seen with endometrial biopsy.20
That being said, sarcomas can be missed, especially in younger women who have extremely large, degenerated, or necrotic-appearing fibroids. Improvements in diagnostic testing are desperately needed to help distinguish benign fibroids from sarcomas, as there are no reliable modalities to exclude a sarcoma at this time. MRI appears to be the most useful imaging modality, although it cannot definitively distinguish a fibroid from a sarcoma. However, a fairly constant finding in leiomyosarcomas is the absence of calcifications. Further, some studies also suggest that ill-defined margins are consistent with a sarcoma. Finally, several centers, including our own, are studying novel biologic markers and revisiting the utility of previously described markers such as LDH in the preoperative detection of uterine sarcoma.21
The way forward
OBG Management: You have said, “Keep patients informed and safe but avoid being too reactionary.” Could you expand on this statement?
Dr. Fader: Certainly. There are many examples throughout the history of medicine in which treatments have brought benefit to thousands or millions of individuals but may cause harm in a select few. We know that when controversial medical issues have arisen in the past, the pendulum has swung widely in terms of societal response.
For example, the landmark Women’s Health Initiative had an immediate and adverse impact on hormone replacement therapy (HRT) administration. The increased risk of cardiovascular disease and breast cancer observed with use of long-term combination therapy with conjugated equine estrogen and medroxyprogesterone acetate prompted many US health-care providers to abandon use of HRT—until more contemporary data demonstrated that, in younger, healthier postmenopausal women, these adverse events were very rare and the benefits of HRT outweighed the risks.
Can we avoid stroke, heart attack, and breast cancer in all younger women taking HRT? Of course not. But we counsel women about the benefit/risk ratio of the therapy and advise them that the likelihood of these events is rare.
Similarly, as with the HRT analogy, younger women have lower risks associated with surgery and morcellation, compared with older women, and are more likely to derive benefit from the procedure (after ensuring appropriate candidacy with a comprehensive preoperative evaluation and informed consent).
However, if the expectation is that no cases of harm will ever occur with a surgical device or procedure in order for it to be deemed acceptable and safe to use in practice, then that is simply an impossible standard to uphold. There is no device, medication, or intervention I know of in medicine that is completely risk-free.
OBG Management: Are there other examples of this type of benefit/risk assessment?
Dr. Fader: Yes. For instance, tamoxifen is a nonsteroidal anti-estrogen agent approved by the FDA for adjuvant treatment of breast cancer, treatment of metastatic breast cancer, and reduction of breast cancer incidence in high-risk women. Tamoxifen has effectively reduced breast cancer rates and significantly improved survival in select breast cancer patients. Yet, it is well known that long-term use of tamoxifen is associated with a twofold increased risk of uterine cancer and uterine sarcoma—a likely far more commonly occurring adverse event than an occult, morcellated uterine sarcoma during a minimally invasive gynecologic procedure.
Should the FDA ban or significantly curtail the use of tamoxifen? No, not likely, because the benefits far outweigh the risks in previvors and hormone-positive breast cancer survivors. “Keep patients informed and safe but avoid being too reactionary” means that we must do our due diligence as physicians by comprehensively counseling and obtaining informed consent from our patients before performing medical interventions. We also must closely scrutinize and improve upon practices that may cause harm.

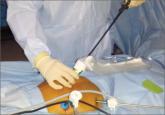
Poor visualization is a criticism of large specimen removal through a minimally invasive approach. This technique improves visualization while...
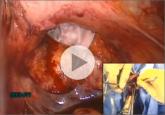
James D. Kondrup, MD, demonstrates his surgical team's approach to morcellation, with use of a morcellation bag after laparoscopic supracervical...
This technique is K. Anthony Shibley, MD's, short-term surgical solution to performing closed power morcellation. His patented pneumoperitoneum...
