Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
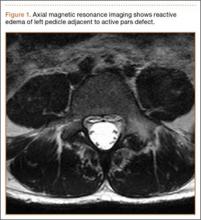
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
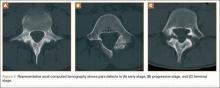
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
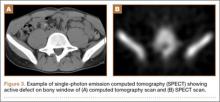
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
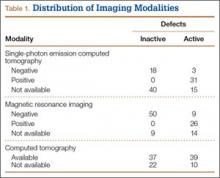
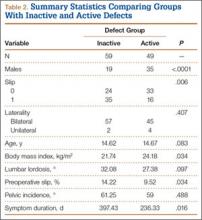
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
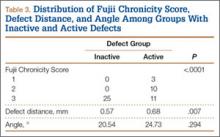
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).