WASHINGTON – Prenatal hydroxychloroquine reduces the risk of cutaneous neonatal lupus by 60% among the infants of women with a systemic autoimmune rheumatic disease.
The medication easily passes the placental barrier and confers significant protection to neonates born to women who have anti-Ro and anti-La antibodies, Julie Barsalou, MD, said at the annual meeting of the American College of Rheumatology.
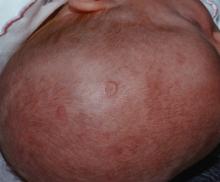
There is currently no therapy for cutaneous neonatal lupus erythematosus (cNLE). The disorder occurs in about 23% of infants born to women with an autoimmune rheumatic disease and is generally benign, said Dr. Barsalou of the Hospital for Sick Children, Toronto. Some infants, however, do develop persistent telangiectasia or epidermal atrophy as a result. A biopsy of the lesions will show interface dermatitis.Toll-like receptors 7 and 9 have been implicated in the initiation and maintenance of interface dermatitis, and hydroxychloroquine inhibits these receptors. In mouse studies, the drug has led to improvement of this type of dermatitis. Hydroxychloroquine also works well in treating subacute cutaneous lupus, she said, and because it can travel across the placenta, it could be an effective means of preventing this disorder in at-risk neonates.
To examine any potential benefit, Dr. Barsalou looked at three pediatric lupus databases: the SickKids NLE database from Toronto, the U.S. Research Registry for Neonatal Lupus, and the French Registry for Neonatal Lupus.
These registries include infants born to mothers with anti-Ro and/or anti-La antibodies, and a diagnosis of lupus, dermatomyositis, Sjögren’s syndrome, juvenile idiopathic arthritis, or rheumatic arthritis. No infants with cardiac neonatal lupus were included in the study.
In addition to hydroxychloroquine, Dr. Barsalou examined the use of prenatal azathioprine, nonfluorinated and fluorinated steroids, and intravenous immunoglobulin.
The cohort comprised 545 neonates born to 535 mothers. Among these, 112 developed cNLE. The remaining 433 infants were used as controls.
Mothers of both cases and controls were a mean age of 31 years. Among cases, the most common diagnosis was Sjögren’s syndrome (53%), followed by systemic lupus erythematosus (46%). Among controls, the most common maternal diagnosis was SLE (62%), followed by Sjögren’s (31%).
All mothers of cases were positive for anti-Ro antibodies; 72% were positive for anti-La antibodies. Among mothers of controls, 99% had anti-Ro antibodies and 48% had anti-La antibodies.
Mothers of cases took hydroxychloroquine (17%), fluorinated steroids (6%), and nonfluorinated steroids with or without azathioprine (28%). Mothers of controls took hydroxychloroquine (34%), fluorinated steroids (4%), and nonfluorinated steroids with or without azathioprine (44%),
There were significantly more female than male infants in the case group (65%). The median age at rash onset was 6 weeks.
Dr. Barsalou performed several multivariate analyses on the entire cohort, as well as two subgroup analyses: one on infants who developed the cNLE rash within the first 4 weeks of life and one on only the infants of mothers with SLE.
In the primary analysis, maternal anti-Ro and anti-La antibodies more than doubled the risk of an infant developing cNLE (odds ratio, 2.5). The use of hydroxychloroquine decreased this risk by 60% (OR, 0.4). Being a female infant increased the risk by 70% (OR, 1.7).
In the group of infants with early-onset rash, maternal anti-La antibodies more than tripled the risk (OR, 3.5), while the use of hydroxychloroquine decreased the risk by 80% (OR, 0.2).
Among the infants born to women with SLE, concomitant secondary Sjögren’s syndrome increased the risk of cNLE by more than threefold (OR, 3.5). Anti-La antibodies more than doubled the risk (OR, 2.5), and the use of hydroxychloroquine decreased it by 60% (OR, 0.4).
“This is the first study to address the prevention of cutaneous neonatal lupus,” Dr. Barsalou said. “We found that prenatal exposure to hydroxychloroquine is likely protective.”
She had no financial disclosures.
On Twitter @alz_gal