Authors: Shavonne L. Massey, MD and Hannah C. Glass, MDCM, MAS
Test your knowledge of this topic HERE.
Seizures are among the most common signs of neurologic dysfunction in the neonatal period.1 Seizures in the neonate most often represent acute injury to the central nervous system, and, less commonly, are the initial presentation of an epilepsy syndrome. During childhood, the highest risk of seizure is in the first year of life, and within that first year the highest risk is in the neonatal period, which is defined as up to 28 days out of the womb or ≤ 44 weeks’ gestation for preterm neonates.2
Seizures in neonates are associated with adverse short- and long-term outcomes, and the seizures themselves may result in additional brain injury.3–8 These adverse outcomes can lead to financial, social, and emotional costs to the patient and caregivers. As studies have linked seizure burden and outcome, it is important to quickly recognize, diagnose, and treat seizures in neonates. Because clinical identification of seizures is not reliable and seizures in neonates often do not have an apparent clinical correlate, neuromonitoring techniques should be used to accurately diagnose and manage neonatal seizures.9 Table 1 lists common neonatal abbreviations and terms used in this article.
Epidemiology
Seizures are among the most common conditions encountered in the neonatal neurocritical care unit.1 The population-based incidence of seizures in neonates ranges from approximately 1 to 5 per 1000 live births in term neonates (≥ 37 weeks’ gestation), but these estimates are based largely on clinical detection of abnormal movements suspected to be seizure, and the actual incidence of electrographic seizures is not known.10 The incidence of seizures is reported to be up to 10-fold higher in preterm (< 37 weeks’ gestation) and low-birth-weight (< 2500 g at birth) neonates, with estimated incidence inversely proportionate to both gestational age and birth weight.2 The estimated incidence of seizure is 20 per 1000 live births in neonates and up to 57 per 1000 live births in low-birth-weight preterm neonates.2,11,12 Table 2 outlines potential risk factors for neonatal seizures.13,14
Etiology
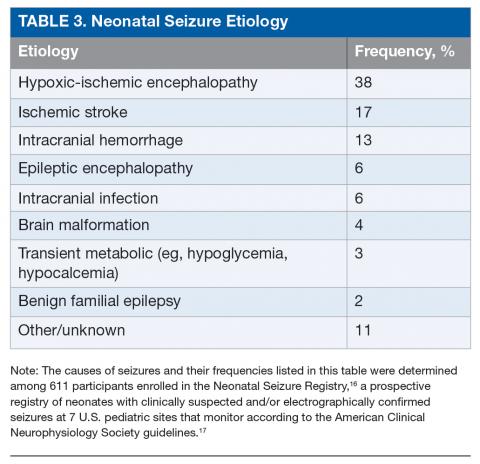
The most common etiology of seizures in neonates is hypoxic-ischemic encephalopathy (HIE). Altogether the acute symptomatic causes, which also include ischemic stroke, intracranial hemorrhage, and, less commonly, infection or transient metabolic abnormalities, account for more than 75% of neonatal seizures (Table 3).15,16 Collectively, the neonatal-onset epilepsies (due to genetic epileptic encephalopathies, benign familial seizures, or brain malformations) comprise a small but important cause of neonatal seizures.16 It is important to distinguish acute symptomatic causes from neonatal-onset epilepsies, since the approach to diagnosis, management, and antiseizure medication choice will differ. Transient metabolic causes of seizures (eg, hypoglycemia, hypocalcemia, and hyponatremia) rarely cause seizure in a tertiary care setting, but must be investigated emergently as correction will often be the only treatment needed.
Test your knowledge of this topic: Board Review Questions
Hypoxic-Ischemic Encephalopathy
HIE is the most common cause of seizures in neonates.15,18,19 Neonates with HIE present with encephalopathy and indicator(s) of a perinatal event (eg, placental abruption, umbilical cord dysfunction), which may include low Apgar scores, acidotic pH, and/or need for advanced resuscitation.20 Seizure onset is typically within the first 24 hours after birth.21,22 Therapeutic hypothermia (which is standard of care for neonates ≥ 36 weeks’ gestation with moderate to severe HIE) has been shown to reduce seizures, but approximately 50% of treated neonates have electrographic seizures nonetheless.23 For this reason, continuous brain monitoring is recommended.17
Ischemic Stroke
The incidence of perinatal arterial ischemic stroke is approximately 10 to 20 per 100,000 live births.24,25 The left middle cerebral artery territory is the most common location of injury, and therefore right-sided hemiclonic seizures (especially in a well-appearing neonate) are a common initial presentation. The etiology is thought to be embolism from the placenta or umbilical cord. Maternal risk factors for arterial stroke include infertility, preeclampsia, prolonged rupture of membranes, and chorioamnionitis.25,26 Infant risk factors are congenital cardiac abnormalities (and especially need for balloon atrial septostomy), systemic and intracranial infection, thrombophilia, and male sex.26,27 Venous strokes occur most commonly in the setting of illnesses, including dehydration and sepsis.28
Intracranial Hemorrhage
Intracranial hemorrhage into the parenchyma or extra-axial spaces, most commonly intraventricular and subarachnoid, can cause seizures (small subdural hemorrhages are common and rarely symptomatic). Intraventricular hemorrhage is the most common cause of seizures in preterm neonates.12,29 Parenchymal hemorrhages may be due to trauma, vascular malformation, cerebral sinovenous thrombosis, or coagulopathy, although in a large proportion, the cause is unknown.30,31
Central Nervous System Infections
Congenital and postnatal central nervous system infections are a rare cause of seizures in neonates. Infection can be acute or chronic and viral (eg, herpes simplex virus, parechovirus, and disseminated enterovirus) or bacterial (eg, group B streptococcus and Escherichia coli).
Brain Malformations
Brain malformations (eg, polymicrogyria, holoprosencephaly, schizencephaly, and lissencephaly, among others) may cause epilepsy with onset in the neonatal period. Neonates with brain malformations can also have seizures due to comorbid HIE and/or electrolyte disturbances or hypoglycemia due to pituitary dysfunction.16
Neonatal-Onset Genetic Epilepsy Syndromes
Neonatal-onset genetic epilepsy syndromes can be benign or malignant. KCNQ2/3 voltage-gated potassium channel mutations were recently recognized as a cause of both benign and malignant neonatal seizure syndromes.32 Benign neonatal familial epilepsy is an autosomal dominant disorder characterized by seizures that typically arise in the first days of life, are easily controlled with antiseizure medications, and resolve within the first year of life. Neonatal-onset epileptic encephalopathies due to KCNQ mutations occur sporadically. Seizure onset is within the first days of life, electroencephalography (EEG) background is abnormal (typically a burst suppression pattern), and seizures can be difficult to control.33 The seizures may resolve in infancy or childhood, but children are typically left with severe global impairments.34 Interestingly, focal tonic seizures are the predominant semiology in both the benign and malignant syndromes. Other genetic causes of early-onset epilepsy syndromes include pyridoxine-dependent epilepsy (ALDH7A1, PNPO) and benign familial infantile epilepsy (PRRT2/KCNT2). Early infantile epileptic encephalopathy (Ohtahara syndrome) and early myoclonic epilepsy have been associated with multiple genetic abnormalities including ARX, CDKL5, and STXBP1 mutations. There is increasing evidence that clinical epilepsy syndromes may be caused by multiple genetic defects, whereas different defects in the same gene may cause diverse phenotypes.
Other Causes
Very rare causes of seizures in neonates include inborn errors of metabolism (eg, urea cycle defects, organic acidurias, and aminoacidopathies), disorders of neurotransmitter metabolism (eg, pyridoxine-dependent epilepsy, nonketotic hyperglycinemia), disorders of energy metabolism (eg, mitochondrial disorders, GLUT1 glucose transporter deficiency, molybdenum cofactor deficiency, and isolated sulfite oxidase deficiency), and biosynthetic defects causing brain malformation or dysfunction (eg, peroxisomal biogenesis disorders). Maternal selective serotonin reuptake inhibitor (SSRI) and serotonin–norepinephrine reuptake inhibitor (SNRI) use during pregnancy may be associated with clinical convulsions in the first hours after birth (SSRI) and electroclinical seizures (SNRI) starting in the first 3 days after birth.35,36 Convulsions without EEG correlate need not be treated with antiseizure medications.
Pathophysiology
Neonates are particularly susceptible to seizures. This increased susceptibility to seizures can be attributed to the risk for trauma during delivery as well as to multiple age-dependent mechanisms.37–39 Enhanced excitability is related to the paradoxical excitatory effect of gamma-aminobutyric acid (GABA) in immature neurons, developmental differences in the glutamatergic system, and delayed maturation of inhibitory systems (Table 4).
Acute symptomatic seizures may harm the developing brain. Studies using animal models show that young animals are more resistant to hippocampal necrosis as compared to adult animals who are subjected to seizures, but hyperthermia and seizures are associated with hippocampal necrosis.40 Additionally, developmental alterations in neuronal circuitry are evident even in the absence of necrosis; early seizures can lead to changes in learning and memory through mechanisms that include altered hippocampal signaling and plasticity, decreased neurogenesis, and delayed neuronal loss.41–44 In animal models, neonatal seizures are also associated with a higher risk of epilepsy later in life.45
In humans, the developmental effect of seizures is difficult to distinguish from the effect of the underlying brain injury, but there is emerging evidence that seizures may have a similar effect in humans as in animal models. Neonates with HIE and seizures have higher lactate peak on magnetic resonance spectroscopy, a finding that is independent of the severity of brain injury.46 Furthermore, children with HIE and early-life seizures also have worse developmental outcomes, and again this finding persists after adjusting for the severity of brain injury.47 Finally, early-life seizures are an important risk factor for remote seizures in children with perinatal stroke.48
Diagnosis
Seizure Definitions
There are 3 types of seizure in the neonate: clinical only, electroclinical, and EEG only (Table 5).
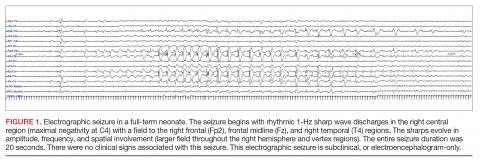
A clinical-only seizure consists of a sudden abnormal clinical change without a coinciding EEG change. On EEG, a seizure is characterized by a sudden abnormal event with a repetitive and evolving pattern that has a minimum peak-to-peak voltage of 2 μV and lasts > 10 seconds (also called an electrographic seizure, Figure 1). An electroclinical seizure consists of a clinical seizure that is simultaneously paired with an electrographic seizure. An EEG-only seizure is a clear electrographic seizure that does not have any associated outwardly visible signs. Neonatal status epilepticus is defined as the summed duration of seizures comprising more than 50% of an arbitrarily defined 1-hour epoch, and thus EEG monitoring is required to make this diagnosis.49
Test your knowledge of this topic: Board Review Questions
Clinical Seizure Semiology
The diagnostic strategies used to identify neonatal seizures have evolved over time. Early studies of neonatal seizures were based solely on clinical observation. Seizures were defined as a paroxysmal alteration in neurologic function that may be temporally associated with electrocerebral changes.50 The most widely accepted scheme for clinical seizures is that proposed by Volpe, in which neonatal seizures are classified as clonic, tonic, myoclonic, or subtle.50 Seizure semiologies have varying concordance with electrophysiology studies. Interestingly, clonic seizures are most reliably associated with an electrographic seizure but are much less common than subtle seizures, which are the least likely clinical seizure type to be associated with an electrographic seizure.51 Generalized tonic–clonic seizures are generally not seen in neonates due to incomplete myelination and limited ability of the neonatal brain to generate a generalized seizure. A modern cohort study involving 647 neonates with video EEG recording examined 160 electrographic seizures in 43 neonates. Myoclonic seizures did not occur. Clonic and tonic seizures occurred in 23% and 25% of the electroclinical seizures, respectively. Subtle seizures were common, with abnormal ocular movements in 70%, orolingual movements in 56%, hypomotor movement in 28%, and autonomic changes in 56%.52 Modern definitions of seizure consider only those that have an electrographic correlate.49
It has become increasingly apparent that clinical observation for seizure detection is insufficient because it has the potential to both overestimate and underestimate the actual seizure burden of the neonate.9 Given the inconsistent correlation between the various described semiologies and electrographic seizures, clinical events noted at the bedside may easily be mistaken for seizure. Indeed, studies have shown poor interrater agreement regarding clinically diagnosed neonatal seizures.9,53 In addition, the bedside clinician will miss seizures that are subclinical (EEG-only) or have subtle manifestations. As a result, EEG use is the gold standard for seizure detection in neonates. The American Clinical Neurophysiology Society (ACNS) provides guidelines for standardized terminology and evaluation of EEG in neonates.49
Neuromonitoring Guidelines
There are 2 primary guidelines for EEG monitoring in the neonatal population. The World Health Organization’s “Guideline on Neonatal Seizures” was created by a multidisciplinary international group of experts with the intention of providing information and recommendations for widespread use of EEG monitoring.54 Strong recommendations include:
- all clinical seizures should be confirmed by EEG where available;
- all electrographic seizures, even without clinical symptoms, should be treated in facilities where EEG is available;
- clinical seizures should be treated if they are prolonged (> 3 minutes) or occurring in clusters.
The ACNS published its “Guideline on Continuous Electroencephalography Monitoring in Neonates” in 2011.17 The document is a consensus statement from neurophysiology experts for standardizing and optimizing neuromonitoring strategies for neonates. To date, this is the most comprehensive guide on neonatal neuromonitoring. Per the ACNS guideline, there are 2 primary indications for EEG monitoring in neonates: (1) to evaluate for electrographic seizures and (2) to judge the severity of an encephalopathy. In terms of seizure detection, the EEG should be used to:
- determine whether a paroxysmal, sudden, repetitive, inexplicable event is a seizure;
- evaluate for the presence of EEG-only seizures;
- evaluate for subclinical seizures while weaning antiseizure medications;
- characterize burst suppression, an electrographic pattern that (a) can be seen in the setting of brain injury, certain metabolic encephalopathies, or genetic syndromes and (b) is used to guide therapeutic intervention in medically refractory epilepsy cases.
EEG is paramount in the evaluation of abnormal paroxysmal events to determine whether they have an electrographic correlate. In addition to the aforementioned difficulties with clinical diagnosis of seizures, neonates have a high rate of EEG-only seizures, with incidences ranging from 10% to 79% across various neonatal cohorts.55–57 These high rates of EEG-only seizures appear to be partially due to the phenomenon of electroclinical dissociation, or electromechanical uncoupling. In electroclinical dissociation, a clinical seizure triggers treatment with an antiseizure medication, but following treatment clinical signs of the seizure disappear while the electrographic seizure continues. Electroclinical dissociation occurs in roughly 50% of neonates.58
The second purpose of EEG monitoring in the neonate is to assess the degree of encephalopathy. The EEG serves as a measure of the neonate’s cortical health. The neurological examination during the neonatal period can be limited by both intrinsic and iatrogenic factors, and many of the activities tested in the neonate (eg, gross movements, the ability to orally feed, the ability to breathe, and the presence of primitive reflexes) are largely measures of brainstem function or spinal reflexes rather than cerebral cortical function. A neonate could potentially have a large supratentorial insult and still accomplish many of the tasks of the neonatal neurologic examination. The EEG is, therefore, an important functional measure of cerebral health in the neonate, and acts as an extension of the neonatal neurologist’s physical examination.
EEG background assessment is also predictive of both short-term outcomes (eg, risk of seizures) and long-term neurodevelopmental outcomes. Interest in using the EEG as a predictor of short- and long-term outcomes is growing, as there is increasing evidence that clinical variables can have limited predictive capability.23 A 2006 study showed that the combination of low Apgar score, low pH, and need for intubation had a positive predictive value of only 25% and negative predictive value of 77% for acute seizure.59 While these features seen immediately after birth are not predictive of seizure, the persistence of certain features, such as lactic acidosis, are more predictive of acute seizure, with longer times to normalization positively associated with higher seizure burden.9 Numerous studies, on the other hand, have shown that a normal or mildly abnormal EEG background is associated with a favorable outcome, while a low-voltage or inactive background is associated with death or significant neurodevelopmental disability.49 A 2016 systematic review of the predictive ability of EEG background features in neonates with HIE examined studies from 1960 to 2014. The review concluded that the appearance of burst suppression (sensitivity 0.87, specificity 0.82), low voltage (sensitivity 0.92, specificity 0.99), and a flat EEG tracing (sensitivity 0.78, specificity 0.99) were most predictive of adverse neurodevelopmental outcomes.60 Neonates with early recovery of EEG background (within 24–36 hours) may be spared adverse outcomes.61,62 A 2014 multicenter study evaluating clinical and EEG risk factors for 90 full-term neonates with HIE found that the initial EEG background predicted subsequent seizure occurrence (excessively discontinuous background with relative risk 17.5; severely abnormal background with relative risk 13) more accurately than clinical variables.23
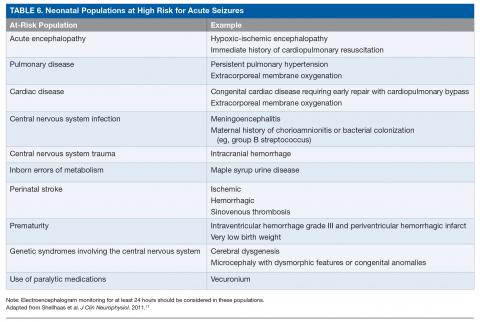
The ACNS guideline also provides more specific details regarding how neuromonitoring should occur. Any neonate receiving an EEG should have at least 1 hour of recording to allow for a full cycle of wakefulness and sleep. At-risk neonatal populations (Table 6) should be monitored for at least 24 hours with EEG to screen for EEG-only seizures, even in the absence of clinically concerning paroxysmal movements. The vast majority of acute seizures in high-risk neonatal groups will occur in the first 24 hours, with nearly 100% occurring within 72 hours of the insult.21,57,63–66 If seizures are detected, the neonate should be monitored until there is no further evidence of seizure on EEG for at least 24 hours. If there are multiple abnormal paroxysmal events of concern, EEG monitoring should continue until all of the events in question are captured.
Test your knowledge of this topic: Board Review Questions
A subsequent report from the ACNS published in 2013 details the exact features of the EEG that should be evaluated in neonates.49 The specific features that are to be assessed in each neonatal EEG include behavioral state, EEG background features, the presence or absence of normal graphoelements, the presence of EEG transient patterns, and the presence of seizures and status epilepticus (Table 5).
Neuromonitoring Modalities
There are 2 primary EEG modalities utilized in the neonatal intensive care unit (NICU): conventional EEG (cEEG) and amplitude-integrated EEG (aEEG).
Conventional EEG. Also called continuous EEG or video EEG, cEEG employs the standardized International 10-20 System of electrode placement with additional electrocardiogram (ECG), respiratory, eye (electrooculographic [EOG]), and electromyography (EMG) channels. cEEG is the gold standard for EEG monitoring in the neonate (Figure 2). It allows for coverage of the entire cerebral landscape, and use of the supplemental channels helps the electroencephalographer decipher cerebral abnormalities from artifactual changes. Additionally, while the patient’s behavioral state is often obvious in adult and pediatric EEGs, behavioral state is notoriously difficult to decipher in neonatal EEGs, given that cerebral patterns of wakefulness and sleep can have similar electrographic appearances in the neonate. The addition of the supplementary channels (ECG, respiratory, EOG, and EMG) adds context to the cerebral patterns to help the neonatal electroencephalographer interpret behavioral state.
While cEEG is the most comprehensive neuromonitoring strategy with the highest yield for accurate seizure detection, it has drawbacks. It is a costly and labor-intensive procedure, requiring trained technologists to apply and set up the EEG, and trained neurophysiologists to interpret the recorded data. This process can lead to delays in the application of the EEG, recognition of seizure on EEG, and subsequent intervention on actionable EEG changes. There have, therefore, been attempts to adapt other modalities, such as quantitative analyses and trending, for bedside use.
Amplitude-integrated EEG. The most commonly employed alternative EEG strategy in the NICU is aEEG, which is a bedside tool that uses a limited recording strategy. A reduced montage of 2 to 4 channels records electrical signal, which is then transformed based on a specific factor (such as amplitude) and displayed on a compressed timescale ranging from 2 to 24 hours (Figure 3). Leads are often placed in the bilateral central or parietal regions for maximal seizure detection, given that the centrotemporal region is the most common location for neonatal seizures.67 The aEEG is typically applied and interpreted by the bedside neonatologist or nurse. This rapid application and interpretation feasibly leads to more rapid intervention. aEEG has an established and validated role in assessment of encephalopathy, particularly in HIE.68 Given the reduced number of recording channels, aEEG is less accurate than cEEG for detecting seizures. While aEEG can accurately identify the binary presence of any seizures in a neonatal EEG record, it largely underestimates the true seizure burden.69,70 aEEG often misses seizures that are composed of slow frequencies and/or low amplitudes and are brief in duration. Seizures can also be missed depending on electrode placement in relation to the location of the seizure.71 aEEG is also subject to false positives, as artifacts can be misinterpreted as cerebral abnormalities. The aEEG lacks the video, EMG, eye, respiratory, and ECG leads that aid the electroencephalographer in deciphering between artifact and cerebral abnormality on cEEG. Lastly, confidence and comfort in aEEG interpretation is variable and often affected by experience and exposure. Survey data suggest a general lack of confidence in aEEG interpretation.72
Despite its limitations, aEEG is being increasingly used in NICUs around the world. A recent survey of U.S. neonatologists found that 55% of respondents use aEEG in their NICU, most often for neonates with hypothermia/HIE (95%) and/or suspected seizures (75%). aEEG was most commonly used to make decisions regarding seizure treatment (~80%), to make decisions regarding therapeutic hypothermia initiation (~50%), for counseling and prognosis (~50%), and to aid in making decisions regarding medication dosages and treatment duration (~35%).73 The ACNS specifically notes that cEEG is the gold standard for seizure detection in the neonate.17 However, recognizing that aEEG use is increasing, the authors comment that aEEG can be used as a supplemental neuromonitoring strategy, particularly in clinical settings where cEEG access is limited. Given the issues with aEEG diagnosis and characterization of neonatal seizures, if seizures are suspected using aEEG, they should be confirmed on cEEG.
Test your knowledge of this topic: Board Review Questions
Treatment
There are no widely accepted guidelines for seizure management in neonates. Optimal treatment of seizures involves rapid identification of the underlying cause (as discussed above, seizures are most often symptomatic of an underlying brain injury, with transient metabolic and early-onset epilepsies as rarer causes). In the acute setting, seizures should be treated as a medical emergency. Reversible causes such as hypoglycemia and hypocalcemia must be immediately evaluated and treated. If infection is suspected, appropriate cultures should be drawn and treatment with antibiotics and/or antivirals initiated. Urgent evaluation of patient and family history, ancillary testing such as EEG and imaging studies, and laboratory tests are important to determine whether the seizures are due to an acute symptomatic cause or an early-onset epilepsy, as the treatment approach differs for each.
Treatment of Acute Symptomatic Seizures
The primary goal of acute symptomatic seizure treatment is to rapidly titrate medications to abolish EEG seizures (including seizures without clear clinical correlate) with the goal of minimizing seizure burden. Acute symptomatic seizures usually begin within 24 to 48 hours after birth (or the acute event) and resolve within 2 to 4 days.65 Since seizures persist after the first dose of medication in more than 50% of neonates, it is important to continue to monitor by EEG for recurrent seizures for at least 24 hours. There are no guidelines to direct the selection of antiseizure medication. A single trial showed that phenobarbital and phenytoin (each given as a bolus dose of 20 mg/kg) had equal efficacy.74 Phenobarbital is the most commonly used initial medication in multiple international surveys and studies.15,75–77
Levetiracetam is a safe alternative that is used widely, although randomized efficacy data are lacking.15,78,79 A large randomized controlled trial comparing phenobarbital and levetiracetam for first-line treatment of neonatal seizures was recently completed (NeoLev2). Preliminary results demonstrate a significantly higher rate of seizure cessation with phenobarbital administration, but fewer side effects with levetiracetam administration. Final results are pending publication. Midazolam infusion is a reasonable alternative or add-on agent for refractory seizures and status epilepticus.80,81
Maintenance antiseizure medications can safely be discontinued in the neonatal period.82,83 For most patients, treatment for 24 to 72 hours after resolution of the acute symptomatic seizures is safe. For neonates without confirmed electrographic seizures (and an adequate monitoring period to capture the events and/or 24 hours seizure-free), maintenance dosing with antiseizure medications may not be necessary, as the likelihood of either nonepileptic events or resolution of seizures is high.
Treatment of Neonatal-Onset Epilepsy
Neonatal-onset epilepsy should be considered when a child has confirmed EEG seizures and an acute symptomatic cause is not found. The approach to treating epilepsy is different from the approach to treating acute symptomatic seizures: medications can be carefully titrated to maximally tolerated doses to determine efficacy and must be continued after discharge home even if seizures are well controlled with antiseizure medications. If no acute symptomatic cause of seizures is identified, a trial of pyridoxine (100 mg intravenously [IV] while EEG is recording), folinic acid (2.5 mg IV), and pyridoxal 5’-phosphate (60 mg/kg/day divided 3 times daily for 2–3 days) is warranted while genetic testing for underlying vitamin-dependent epilepsies is pending.84 For neonates with suspected KCNQ2/3 epilepsy (either benign or malignant), carbamazepine or oxcarbazepine is indicated as the first-line agent, with retigabine as an alternate agent.85 Neonates with focal seizures due to brain malformation may also respond to carbamazepine/oxcarbazepine. Table 7 lists the most commonly used antiseizure medications in neonates.
Test your knowledge of this topic: Board Review Questions
Outcomes
Both animal and human data suggest that seizures can negatively impact the developing brain. As noted in the Pathophysiology section, preclinical studies suggest that the immature brain is more susceptible to seizures, and that seizures during early life may result in the development of inappropriate cerebral electrical pathways, which can beget epileptic networks later in life.86 Clinical data have been less definitive, as the link between poor outcomes and seizure is complicated by the underlying etiology and, possibly, interventions. Typical outcome measures assessed in neonatal seizure populations are neuroimaging, neurodevelopment, and occurrence of remote epilepsy. Several studies have shown a correlation between seizure burden and worsened magnetic resonance imaging (MRI) scores, particularly in neonates with HIE.4,21,63 The sheer presence of electrographic seizures is associated with acute MRI injury, with higher seizure burden correlating with more severe MRI injury. The association between seizures and MRI injury does not appear to vary with seizure type (electroclinical versus EEG only).21 In neonates with HIE, those with seizures are more likely to have cortical or near-total brain injuries seen on MRI as compared with those without seizures.21
Neurodevelopmental measures are consistently worse in children with a history of neonatal seizures compared with healthy peers or populations with neonatal brain injury without seizure. A prospectively assembled cohort with clinically diagnosed neonatal seizures followed for a median of 10 years in Newfoundland, Canada, has provided some of the most informative longitudinal data on such patients.8 Children born at term do better than children born prematurely, but increased rates of morbidity and mortality are present in both groups. During the 10-year follow up period, 16% of term neonates and 42% of preterm neonates died. Among survivors, impairments were seen in 39% of term neonates and 46% of preterm neonates at follow up. The most common impairments were epilepsy (27%), learning disabilities (27%), cerebral palsy (25%), and intellectual disability (20%). Predictors of poor outcome included severe encephalopathy, cerebral dysgenesis, complicated intraventricular hemorrhage, infections in preterm neonates, abnormal EEG, and requiring multiple antiseizure medications.
Other studies have found that the presence of neonatal seizures is associated with development of microcephaly, cerebral palsy, and failure to thrive, particularly in subsets of children with HIE.7 In addition, studies have suggested a relationship between seizure burden and developmental outcomes, with increasing seizure burden associated with worse neurodevelopmental outcome. A study of a heterogenous group of 56 term neonates with status epilepticus found that 75% had poor outcomes, defined as a developmental quotient less than 85 at 18 months of age or later.87 In a subset of patients with HIE, the duration of status epilepticus was predictive of poor neurodevelopmental outcomes, with neonates with poor neurodevelopmental outcomes having a median of 215 minutes of seizure and those with good neurodevelopmental outcomes having a median of 85 minutes of seizure. Others have studied the impact of neonatal seizures on intelligence quotients (IQ), finding that the presence of high clinical and/or EEG seizure burden in the setting of HIE was associated with substantially lower full-scale IQ scores (96.9 in no seizure, 82.7 in mild/moderate seizures, 67.2 in severe seizures), which was maintained after adjusting for MRI severity.47 Additionally, the absence of seizures has been shown to be an independent predictor of improved 18-month outcomes, defined as lack of death or disability, in asphyxiated neonates treated with hypothermia.88
The risk of epilepsy following neonatal seizures is also increased compared to the general population. A 2015 literature review found that in 4538 children with a history of neonatal seizures, 18% developed epilepsy, with nearly 70% having onset within the first year of life.6 Of those patients who developed epilepsy, 81% had an associated neurological impairment (18% with intellectual impairment, 6% with cerebral palsy, and 45% with both cerebral palsy and intellectual impairment). Additionally, population studies of children with epilepsy have shown that a history of neonatal seizures decreases the likelihood of later seizure freedom.89
Conclusion
The risk of brain injury is high in the perinatal and neonatal period. Seizures, which are the most common manifestation of cerebral injury during the neonatal period, are therefore relatively common. Neonatal seizures most often represent an acute cerebral injury, but can also be the result of a developmental brain abnormality or genetic epilepsy, and herald risk of continued or recurrent seizure. Although there is a long list of potential causes of neonatal seizures, by far the most common cause of seizure in the term neonate is HIE. The only intervention for this entity, therapeutic hypothermia, leads to improved neurodevelopmental outcomes and appears to lower the seizure burden. It is important for the practitioner to be mindful of potential other causes for neonatal seizures, particularly when there is no history of a clear asphyxial event, as these other etiologies may require etiology-specific treatments and may confer different prognoses. There are several populations considered high risk for neonatal seizures, and neuromonitoring with cEEG should be strongly considered in these patients given high rates of subclinical seizures.
When they occur, neonatal seizures are frequent, typically occur within the first 48 hours following insult, are often subclinical, and most often have a centrotemporal onset. Seizures are classified as clinical only, electroclinical, and EEG only depending on the presence and relationship of paroxysmal abnormal movements with defined changes on the EEG. Although traditionally the diagnosis of seizure was made on a clinical basis, it is now well established that the clinical diagnosis of seizures will both overestimate and underestimate the true incidence of seizure. As a result, EEG is required for the diagnosis of neonatal seizures. cEEG remains the gold standard for neonatal neuromonitoring, although adapted montages such as aEEG can act as a complementary bedside tool for more rapid seizure management.
The mainstays of treatment for neonatal seizures are phenobarbital, phenytoin, and benzodiazepines. These medications are the only treatments that have been studied in a randomized fashion with published results. None of these treatments are ideal, as they are at best moderately effective, all have side effects that can be dose-limiting, and their prolonged use may be harmful. Newer-generation medications such as levetiracetam are being used with increasing frequency, although safety and efficacy data are limited. Given the relationship between neonatal seizures and neurodevelopment, mortality, and the development of epilepsy, it is important that we continue to strive to find the ideal intervention strategy for these youngest and most vulnerable members of society.