Feature
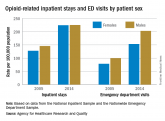
For opioid-related hospitalizations, men and women are equal
The gender gap increased in emergency department visits.

A concerted, multiagency, multipronged counterattack is needed to break the opioid epidemic’s grip on the nation, according to a new report from the National Academies of Sciences, Engineering, and Medicine.
The report, commissioned by the Food and Drug Administration to strengthen its own 2016 Opioids Action Plan, presents a national strategy for addressing this “urgent public health priority.”
“The ongoing opioid crisis lies at the intersection of two substantial public health challenges – reducing the burden of suffering from pain and containing the rising toll of the harms that can result from the use of opioid medications,” according to a summary of the report, “Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use.”
The National Academies’ Committee on Pain Management and Regulatory Strategies to Address Prescription Opioid Abuse spent more than a year studying the matter and developing the report. Among its recommendations is a call for investment in research to better understand pain and opioid use disorder.
“We have a specific lack of knowledge about how pain and opiate use disorder [OUD] interact – that is, why patients in pain might be more or less at risk for the development of opiate use disorder when exposed to either illicit or prescription medication,” committee member David Clark, MD, professor of anesthesia, perioperative pain medicine, and pain at Stanford (Calif.) University, said during a webinar on the report.
“This leads up to one of our first recommendations, which is to invest in research to better understand pain and opiate use disorder,” Dr. Clark said. “We want to emphasize that we need to improve our understanding of the neurobiology of pain,” which will, hopefully, provide a better foundation for the development of nonopioid treatments.
The committee also recommended that the FDA:
“The committee believes a commitment to transparency is critical to maintain balance between preserving access to opioids when needed and mitigating opioid-related harms and to maintain public trust,” the report’s authors said. “The committee also believes aggressive use of the FDA’s currently available authorities, such as Risk Evaluation and Mitigation Strategies (REMS), safety labeling changes, and risk communications is critical to supporting the safe and effective use of opioids.”
The committee also recommended strengthening the postapproval oversight of opioids.
A full review of currently marketed and approved opioids was also recommended, as was the application of public health consideration to opioid scheduling decisions.
“The particular characteristics of opioids, we believe, require a certain amount of what we term ‘opioid exceptionalism’ from the regulator in terms of trying to consider the larger societal effects of approval and use of opioid medication,” committee member Aaron S. Kesselheim, MD, JD, of Harvard Medical School, Boston, said during the webinar. “We believe that this public health approach is both reasonable for the FDA, given the fact that it is primarily a public health agency, and can be implemented at all different levels of the development process.”
Strategies recommended by the committee for the FDA, state agencies, health professionals, and relevant private organizations, such as through public-private partnerships, include:
According to the report, containing the opioid epidemic and ameliorating its harmful effects on society through the development of these strategies will require “years of sustained and coordinated effort.”
Indeed, things will get worse before they get better, the committee predicted.
“Trends indicate that premature deaths associated with the use of opioids are likely to climb and that opioid overdose and other opioid-related harm will dramatically reduce quality of life for many people for years to come,” the report’s authors noted. “Access to evidence-based treatment for OUD and efforts to prevent overdose deaths and other harms should therefore be increased substantially and immediately as a public health priority.”

The gender gap increased in emergency department visits.

Food and Drug Administration officials are calling for a sweeping overhaul of the agency’s approach to opioid medications, including renewed...

Nonopioid therapy is the preferred approach to manage chronic pain outside of active cancer, palliative, and end-of-life care, the CDC said.
