SAN FRANCISCO – ordered by the Food and Drug Administration.
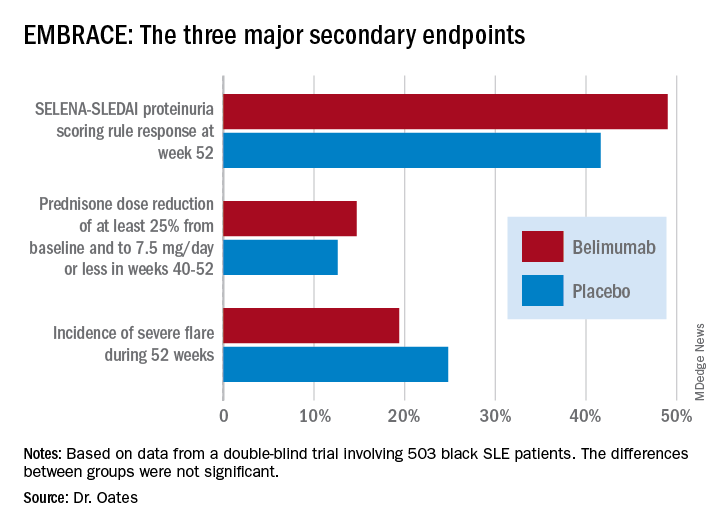
Numerically, the outcome trends in the EMBRACE study consistently favored belimumab (Benlysta) over placebo. And not just for all three components of the primary endpoint, but for each of the three major prespecified secondary endpoints as well. Yet not a single one of those six favorable trends attained statistical significance, Jim C. Oates, MD, reported at an international congress on systemic lupus erythematosus.
“The study did not achieve its primary endpoint, although there was a numeric advantage for patients on belimumab, with a 40% increase in the chance of response,” observed Dr. Oates, professor of medicine, director of the division of rheumatology and immunology, and vice chair for research at the Medical University of South Carolina, Charleston.
EMBRACE was a 52-week, double-blind trial in which 503 black systemic lupus erythematosus (SLE) patients were randomized 2:1 to 48 weeks of IV belimumab at the approved dose of 10 mg/kg or to placebo infusions on top of standard care background therapies. The postmarketing study was required by the FDA as a condition for the agency’s 2011 marketing approval of belimumab, a human monoclonal antibody that inhibits B-cell activating factor, also known as a B-lymphocyte stimulator. The agency request came because the three premarketing phase 3 intravenous trials, as well as the phase 3 subcutaneous belimumab trial, included only small numbers of black patients, and the results in that population were conflicting.
The EMBRACE results are particularly disappointing in light of the increased prevalence, severity, and mortality of SLE in black patients. However, the final word on EMBRACE isn’t in, as the data from the recently completed open-label extension phase beyond 52 weeks have not yet been analyzed.
Roughly three-quarters of EMBRACE enrollees completed the full 52 weeks of study. Withdrawal for adverse events or lack of efficacy occurred in 6.7% and 5.4%, respectively, of placebo-treated controls, and in a respective 5.4% and 4.7% of patients in the belimumab arm.
The primary study outcome at 52 weeks was the SLE Responder Index (SRI) response rate with the modified SLE Disease Activity Index (SLEDAI)–2000 scoring for proteinuria (SRI-S2K). This required at least a 4-point reduction from baseline in the Safety of Estrogens in Lupus Erythematosus – National Assessment (SELENA)-SLEDAI, no worsening in the Physician Global Assessment, as well as no new British Isles Lupus Assessment Group (BILAG) A or two new BILAG B organ domain scores. This outcome was achieved in 48.7% of the belimumab group and 41.6% of controls, for 40% greater likelihood in the active treatment arm in a logistic regression analysis, which didn’t achieve statistical significance. While the between-group difference was significant in favor of belimumab during the monthly assessments at weeks 32-44, the belimumab and placebo response rates converged thereafter.
The belimumab safety profile contained no surprises. Of note, rates of opportunistic infections, depression, and suicide or self-injury, which had been deemed adverse events of special interest based upon previous studies, were numerically lower than in controls.
The deflating EMBRACE results are sure to come under close scrutiny, since black patients with SLE have been identified as a population with a major unmet need for improved therapies. Of note, 44% of study participants were from the United States and Canada, and they had a longer disease duration, lower damage scores, and less serologically active disease than subjects from the rest of the world.
Because the results of prior phase 3 studies showed increased belimumab response rates in patients with serologically more active disease, prespecified subgroup analyses of the composite endpoint were conducted. These analyses parsed out several subgroups who were significantly more likely to achieve the primary endpoint with belimumab than with placebo. Black patients with a baseline SELENA-SLEDAI-S2K score of 10 or greater had a 52.5% response rate to belimumab, compared with 40.9% with placebo, for a 76% relative increase. Patients with a low baseline C3 and/or C4 were 200% more likely to respond to the biologic agent than placebo, by a margin of 47.2% versus 24.6%. And patients from outside North America were 80% more likely to respond to belimumab, with a 57.5% response rate, compared with 44.0% on placebo.
One audience member noted there is evidence that the use of mycophenolate appears to be advantageous in African American SLE patients and wondered if the EMBRACE subgroup on belimumab plus background mycophenolate fared significantly better than with placebo. Dr. Oates replied that, although it’s an important question, the subset analysis isn’t available yet.
The EMBRACE trial was sponsored by GlaxoSmithKline. Dr. Oates reported receiving research funding from that pharmaceutical company and several others, the National Institutes of Health, and the Department of Veterans Affairs.
SOURCE: Oates JC et al. Lupus Sci Med. 2019;6[suppl 1], Abstract 200.