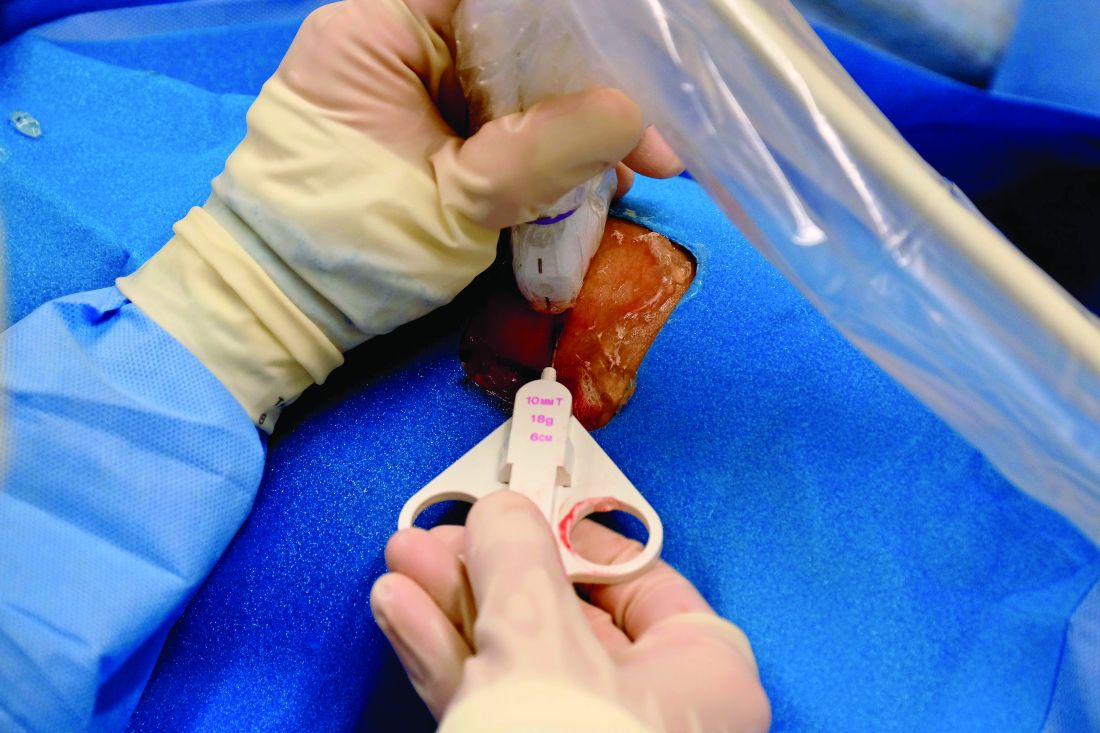
Musculoskeletal ultrasound (US) technology, however, has advanced dramatically over the past decade, is available and used by rheumatologists in clinical practice, and has brought US-guided joint biopsies to the forefront of research. Such techniques have been used in Europe for years, and as a result, an extensive catalog of literature supports the safety, feasibility, and tolerability of the approach.
A recent study in Portugal by Romao et al., for example, showed “remarkably high” patient tolerability (70%) with 64 US-guided procedures, including 52 in clinical practice and 12 for research purposes. No major adverse events occurred, and biopsy usefulness was high, with 37% having a direct diagnostic impact and with 100% and 95% positive- and negative-predictive values for infection. Further, synovial tissues were retrieved in 88% of biopsies and a median of 75% of samples were gradable (Arthritis Care Res. 2019 Aug 17. doi: 10.1002/acr.24050).
A 2018 study of 524 synovial biopsies, including 402 performed using US-guided needle biopsy, performed at five centers across Europe similarly demonstrated safety and patient tolerability (RMD Open. 2018;4[2]:e000799. doi: 10.1136/mdopen-2018-000799).
Building on the work in Europe, investigators at Northwestern launched the REASON study, assembling a consortium of academic rheumatology groups across the United States, training participants in minimally invasive US-guided joint biopsy techniques, and collecting and analyzing synovial tissue samples submitted by the participating centers.
Laura B. Hughes, MD, a professor at the University of Alabama at Birmingham and an investigator in both the REASON study and AMP, said in an interview that her experience with patients is similar.
“It has been very, very well tolerated,” she said of the biopsy procedure used in the course of the studies – and that’s despite the time and commitment required, she added, explaining that 12 samples, each requiring a separate injection, are obtained over a 30- to 45-minute visit.
“We’ve had no problems, no complications,” she said, also noting the importance of careful patient selection.
Patients are altruistic; they want to be a part of moving things forward and helping other patients, and they have been more than willing to participate, both she and Dr. Perlman noted.
In fact, the REASON study investigators reported that performance of STB by rheumatologists in the United States is feasible and generates high-quality samples.
Further, the transcriptional profiles of isolated RA synovial macrophages identified from samples submitted by Dr. Hughes and others in the network characterized subpopulations of patients and identified six novel transcriptional modules associated with disease activity and therapy, underscoring the potential for precision medicine in RA.
“We posit that transcriptional signatures in macrophages ... will predict responsiveness to specific nonbiologic and/or biologic therapies,” they wrote, adding that future studies will “entail collection of synovial biopsy specimens from a larger cohort longitudinally, prior to, and following therapy.”
The ongoing National Institutes of Health–funded AMP Network research is also using synovial biopsies, but more for identification of molecular pathways with a focus on potential drug development.
A 2019 report from the AMP investigators described their integrated use of single-cell transcriptomics and mass cytometry to reveal cell states expanded in RA synovia and the mapping of inflammatory mediators to their source cell populations, which may be key mediators of RA pathogenesis.
“We observed upregulation of chemokines (CXCL8, CXCL9, and CXCL13), cytokines (IFNG and IL15), and surface receptors (PDGFRB and SMAMF7) in distinct immune and stromal cell populations, suggesting potential novel targets,” they wrote (Nat Immunol. 2019 Jul;20[7]:928-42).