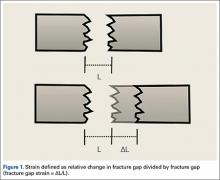
Fracture healing can be categorized as primary or secondary. Primary healing requires precise reapproximation of bone fragments and compression of cortices. Osteons are formed across the fracture line, allowing blood supply and endothelial cells to gain access, leading to osteoblast infiltration and subsequent bone formation.1 This type of bone healing can be accomplished only with absolute stability—specifically, only with less than 2% strain at the fracture site, necessitating operative intervention with compression plating (Figure 1).2 This type of construct generates friction between the bone fragments against a metal plate, created by tightening screws that purchase both far and near cortices of bone.3 Although this type of fixation works well with many fractures, there are several instances in which compression plating is not ideal.4 Osteoporotic bone, for example, limits the amount of compression that can be developed, as screws strip the bone more readily, leading to weakened constructs prone to failure. Metaphyseal fractures in which there is minimal cortex for screw thread purchase are a similar challenge.5 Highly comminuted fractures do not allow for sufficient fragment compression and stability. In addition, compression plating requires periosteal stripping at the fracture, and often substantial soft-tissue disruption, which is especially a problem in areas of tenuous blood supply (eg, the tibia).
Locked plating therefore has become a valuable technique in managing osteoporotic fractures.2 Locking plates may be used to achieve secondary bone healing through a small amount of interfragmentary motion, 0.2 to 10 mm, as seen with bridge plating for example, whereby the locking plates act as internal fixators. Much as with external fixators, as the distance from the fixator bar (or plate) to bone decreases, construct stiffness increases. Thus, locking plates function as extremely stiff fixators when the plate is very near bone. It has therefore been speculated that such stiffness is insufficient to provide optimal secondary healing conditions.6,7 Titanium (vs stainless steel) plates have been used, and screws have been omitted just adjacent to either side of the fracture site, in attempts to increase plate flexibility and thus interfragmentary motion.8,9 In addition, biomechanical and animal model studies have demonstrated that, with use of locking plates, motion at the fracture site is asymmetric and leads to unequal callus formation at the near and far cortices, thus weakening the fracture site.10,11
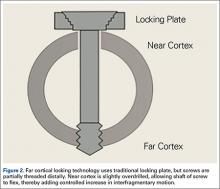
The locking plate design was recently modified to address these concerns. Far cortical locking (FCL) uses locking screws threaded only distally (Figure 2), which allows for purchase into the far cortex but not the near cortex, which increases pin length from plate to bone. The near cortex is no longer anchored to the plate and thus increases construct flexibility. Pilot holes in the near cortex allow for movement of the nonthreaded screw shaft in a controlled, biphasic manner.12 This design decreases stiffness while sacrificing very little construct strength.10 In addition, motion at the far and near cortices is nearly parallel. It has been shown in an ovine tibial osteotomy model that, compared with the traditional locking plate design, FCL generates symmetric callus formation and improved fracture healing.11 Although these results are promising, there are only limited clinical data on use of the FCL technique in fracture repair. Our null hypothesis was that, despite the theoretical advantages of FCL constructs over conventional locking plates, there would be no clinically observed differences between the constructs.
Patients and Methods
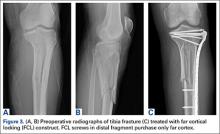
After obtaining Institutional Review Board approval from the 2 level I trauma centers and 1 level II trauma center involved in this study, we retrospectively reviewed the cases of all adults who presented with a tibia fracture and were treated with FCL technology (MotionLoc, Zimmer) by a fellowship-trained trauma surgeon at these hospitals (Figures 3A–3C). Any primary tibia fracture treated with FCL was considered. Only patients with follow-up of at least 20 weeks were included in the analysis. Exclusion criteria were tibial malunions or nonunions treated with FCL and fractures treated with a combination of intramedullary fixation and plating.
We reviewed the patient charts for demographic data, mechanism of injury, fracture type, and comorbidities. Risk factors for poor healing—such as diabetes and tobacco use, either current or prior—were recorded. We also reviewed the radiographs of the initial injuries for analysis of the tibia fracture types (Table 1) as well as the follow-up radiographs for evaluation of fracture healing. Using the Orthopaedic Trauma Association classification system, we identified a variety of fracture patterns. Fracture healing rates were recorded and used to calculate the overall healing rates for each group. Union was defined as either radiographic evidence of a completely healed fracture (≥3 cortices) or radiographic evidence of osseous bridging at the fracture site in addition to full weight-bearing without pain. Infection was defined as positive intraoperative cultures or grossly infected wounds with purulence and erythema.