Take-Home Points
- Adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome following pediatric spine surgery.
- Must recognize nonspecific symptoms such as abdominal pain, tenderness, distention, bilious or projectile vomiting, hypoactive bowel sounds, and anorexia postoperatively.
- Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, or cardiovascular collapse and death.
Superior mesenteric artery (SMA) syndrome resulting from surgical treatment of scoliosis has been recognized in the medical literature since 1752.1 Throughout the literature, SMA syndrome variably has been referred to as cast syndrome, Wilkie syndrome, arteriomesenteric duodenal obstruction, and chronic duodenal ileus.2 We now recognize numerous etiologies of SMA syndrome, as several sources can externally compress the duodenum. Classic acute symptoms of bowel obstruction include bilious vomiting, nausea, and epigastric pain. Chronic manifestations of SMA syndrome may include weight loss and decreased appetite. Our literature review revealed that adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome after pediatric spine surgery.
We report the case of a 14-year-old boy who developed SMA syndrome after undergoing scoliosis surgery. The patient and his mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy with a history of idiopathic scoliosis presented to Cohen Children’s Hospital (Long Island Jewish Medical Center) with bilious vomiting that had persisted for 7 days after posterior T9–L4 fusion with instrumentation.
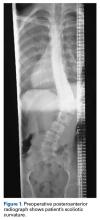
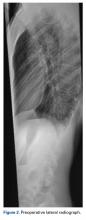
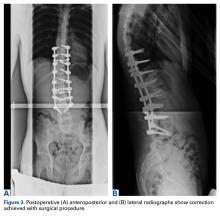
Preoperative radiographs revealed a 55° right Lenke V C curve (Figures 1, 2). Before the procedure, the patient weighed 111.6 lb and was 175 cm tall. The surgery was uneventful, with a curve correction to about 7° (Figures 3A, 3B). No abnormalities were noted during intraoperative neurologic monitoring. After an unremarkable postoperative course, on postoperative day 19 the patient presented to the emergency department (ED) with abdominal pain, nausea, and vomiting of 3 days’ duration. Right lower quadrant ultrasound revealed nonspecific fluid-filled bowel loops, and the patient was discharged with antiemetics and instructions for hydration. Two days later, he returned to the ED with unrelenting brown vomitus and abdominal pain and noted a 20-lb weight loss over 2 weeks. He was admitted to the postanesthesia care unit for dehydration and for QT prolongation secondary to electrolyte abnormalities. On admission, he weighed 88.2 lb. An upper gastrointestinal (GI) contrast radiograph confirmed a diagnosis of SMA syndrome, and a nasojejunal tube was placed. The patient gained no weight over 10 days; a gastrojejunal tube was placed until he was able to tolerate oral nutritional intake, 5 weeks later. He was followed by the nutrition and general surgery teams to ensure clinical improvement. Surgical intervention was unnecessary. One year after surgery, the patient was home and doing well without permanent sequelae.Discussion
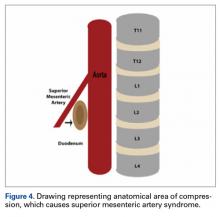
SMA syndrome is attributed to the anatomical orientation of the third part of the duodenum, which passes between the aorta and the SMA (Figure 4).
The SMA, an anterior branch of the aorta at the L1 vertebral level, is encased in fat and lymphatic tissue. Its acute caudal descent is sometimes referred to as a nutcracker configuration.2 Normal SMA angles are highly variable. One study described 75 aortas with angles ranging from 20° to 70°.3 SMA angle reduction results in extrinsic compression of the duodenum by the SMA. A common influence is the loss of protective peripancreatic and periduodenal fat below the SMA origin secondary to significant weight loss of any kind, such as from anorexia nervosa, malabsorption, and malignancy. Correcting a scoliotic curve through spinal manipulation essentially results in a lengthening of the vertebral column, which displaces the SMA origin more superiorly and creates a more acute aortomesenteric artery angle.Adolescents are particularly vulnerable to this condition. Faster adolescent bone growth relative to visceral growth is accompanied by a decrease in SMA angle.3 Occasionally, body casts are used after surgery to immobilize the vertebrae and augment healing. Cast syndrome occurs when pressure from a body cast causes a bowel obstruction secondary to spinal hyperextension and amplified spinal lordosis.2 This finding, dating to the 19th century, was reported by Willet4 when a patient died 48 hours after application of a body cast. In 1950, the term cast syndrome was coined after a motorcyclist’s injuries were treated with a hip spica cast and the patient died of cardiovascular collapse secondary to persistent vomiting.5
Table 1 summarizes various evaluation, diagnosis, and treatment algorithms designed to optimize nutrition and weight in patients developing signs and symptoms of SMA syndrome after posterior spinal instrumentation and fusion for adolescent idiopathic scoliosis (AIS).
Of note, about 50% of patients with SMA syndrome present in the first week after spine surgery, 35% in the second week, and 15% more than 2 weeks after surgery. A patient presenting with abdominal pain/distension, nausea, and vomiting after scoliosis surgery should be initially evaluated for signs of intestinal obstruction.6 An abdominal radiograph can be used to assess for distended bowel gas or air-fluid levels, though this imaging study has also been found to be within normal range in an eventual SMA syndrome diagnosis. SMA syndrome can often be differentiated from postoperative ileus by fever/tachycardia and peritoneal signs. In the presence of positive findings for intestinal obstruction, initial management should begin with nasogastric decompression, electrolyte correction, and intravenous hydration. Otherwise, management should be to observe, treat with antiemetics, and reassess periodically.6 The first step is to start auxiliary enteral nutritional support through a nasojejunal feeding tube—or total parenteral nutrition if enteral feeding is unacceptable. Often, SMA syndrome is definitively diagnosed with an upper GI barium study with simultaneous angiography. If the diagnosis of SMA syndrome is made and symptoms improve, conservative management should be continued and diet slowly advanced. If symptoms worsen or significant weight loss occurs, surgical management should be considered. Surgical management is performed through laparoscopic or open duodenojejunostomy, division of the ligament of Treitz, or a modified Ladd procedure.7-10 Removal of spinal implants and cast is unnecessary, except when lumbar spine hyperextension is the cause, in which case cast and metal implants must be removed to relieve the duodenum from the SMA.7The incidence of SMA syndrome after scoliosis surgery is 1% to 4.7%.3,6,7 Our literature review of SMA syndrome after scoliosis surgery for AIS revealed 19 case reports over 45 years (Table 2). Studies reported that the incidence of SMA syndrome was higher in certain groups based on the extent of spinal deformity and the Lenke classification system for scoliosis.11,12 Specifically, groups with body mass index under the 25th percentile, Lenke B or C (laterally displaced, curved) scoliosis, and stiffer thoracic curves (<60% correction) have a higher incidence.12 Overall, initial presentation of SMA syndrome generally consists of a combination of abdominal pain/distension, nausea, vomiting, and varying degrees of weight loss. Although the predominant cases are confirmed with upper GI contrast studies, some cases are confirmed with radiographs, laboratory (serum lipase) abnormalities, and correlated with their clinical presentation in order to direct their therapy.13-15 For patients diagnosed with SMA syndrome, length of stay varies significantly, from 3 to 71 days. Time in hospital generally depends on ability to transition a patient to oral intake without complication. Eighty-five percent of reported cases of SMA syndrome after spinal surgery for AIS present within the first 2 weeks after surgery.1,6,7,9,13-19Our patient’s case had a combination of unique features. First, he presented 19 days (almost 3 weeks) after surgery. We identified only 3 other case reports in which the patient presented later (most SMA syndrome symptoms present within 2 weeks of the spinal procedure). One patient presented on postoperative day 27 and was discharged with a nasojejunal tube because of an inability to tolerate oral intake.6 Another patient presented 40 days after surgery, underwent laparotomy (a fundal perforation was found), and died immediately afterward.15 A third presented 45 days after surgery and had a treatment experience similar to our patient’s: nasogastric decompression, intravenous fluids, nasojejunal tube feeding, and transition to oral intake before discharge.7Our case’s second unique feature is the 20-lb weight loss over 2 weeks—more than in most other cases over the same period. For patients with recorded weight loss, average weight loss was about 6.2 pounds per postoperative presentation week, and only 1 patient presented with a steeper trajectory of weight loss before presentation.18 Our patient may have waited longer to present to the ED or may have had a more severe case of the disease.The third unique feature in this case is electrocardiogram findings. Although some cases briefly discussed electrolyte abnormalities, none presented evidence that these abnormalities caused cardiac changes.6,16,18 The overall clinical significance of the QT prolongation in our patient’s case is unknown, as this finding was improved with correction of the electrolyte abnormalities and appropriate fluid replenishment.
Early recognition of nonspecific symptoms (eg, abdominal pain, tenderness, distension, bilious or projectile vomiting, hypoactive bowel sounds, anorexia) plays a key role in preventing severe morbidity and mortality from SMA syndrome after scoliosis surgery. Although many patients present in the semiclassic obstructed pattern, notable reasons for diagnostic delay include normal appetite and bowel sounds.3 For example, SMA syndrome may be misdiagnosed as stomach flu because of unfamiliarity with disease diagnosis and management.20 Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, and cardiovascular collapse and death.
Am J Orthop. 2017;46(2):E124-E130. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.