Take-Home Points
- Cellular healing response differs between bony and soft tissue biceps tenodesis.
- Bony tenodesis incites an inflammatory healing response.
- Bony tenodesis healing occurs at the tendon-bone interface.
- Intrasseous bony fixation leads to tendon degeneration within the bone.
- Tendon-to-tendon tenodesis may result in regenerative tendon healing.
The long head of the biceps tendon (LHBT) is a well-established pain generator of the anterior shoulder 1,2 and may be surgically addressed in refractory cases. 3 According to a recent study of 44,932 cases, biceps tenodesis rates increased 80% over just 3 years (2008-2011). 4 Nevertheless, optimal tenodesis location and technique remain controversial. Proximal and distal tenodesis, including numerous soft-tissue and bony techniques, have been described. 5-7 Several studies have focused on the biomechanical strength of various fixation modalities. 8-14 These data highlight the ongoing evolution of our understanding of biceps-labrum complex (BLC) disease.
Over the years, tenodesis location has proved to be an important factor in outcomes. 3,15-20 Several recent studies have elucidated the role of the extra-articular LHBT and the limited capabilities of diagnostic arthroscopy. 15-17,20,21 Taylor and colleagues 17 defined the bicipital tunnel as the extra-articular segment of LHBT and its fibro-osseous enclosure. The tunnel extends from the articular margin through the subpectoral region and can be divided into 3 zones: Zone 1 goes from the articular margin to the inferior margin of the subscapularis, zone 2 goes from the inferior margin of the subscapularis to the proximal margin of the pectoralis major tendon, and zone 3 is the subpectoral region. Zone 2 is often referred to as “no man’s land” for its relative invisibility from arthroscopy above and open exposure below. 17,21 Notably, a recent study reported a 47% prevalence of hidden tunnel lesions in patients with chronic BLC disease symptoms. 18 Other studies have shown that standard proximal tenodesis methods often fail to address LHBT pathology in this area, leading to residual symptoms. 9,22 It is evident that tenodesis location and technique play important roles in patient outcomes. Sanders and colleagues 16 found that the revision rate was significantly higher among patients who underwent biceps tenodesis without release of the bicipital tunnel sheath than among patients who underwent tenodesis with the release. Dr. O’Brien developed an alternative option: soft-tissue tenodesis with transfer of the LHBT to the conjoint tendon within the subdeltoid space. 23,24 This technique addresses intra-articular and extra-articular tunnel disease while mitigating the complications associated with bony tenodesis. Early and midterm studies have shown this to be an effective intervention for chronically symptomatic BLC disease. 25,26
Despite the abundance of literature on tenodesis techniques, no one has histologically evaluated the location-dependent healing and inflammatory responses. We conducted a study to determine the impact of tenodesis location on healing and inflammation in a rat model. We hypothesized that, compared with tendon-to-bone techniques, soft-tissue tenodesis would minimize inflammatory response and optimize healing.
Methods
The study was approved by the Institutional Animal Care and Use Committee at the Hospital for Special Surgery.
Animals
Biceps tenodesis was performed at 1 of 3 locations in 36 thirteen-week-old Sprague-Dawley rats (Charles River Laboratories). All rats were prepared for surgery by an experienced veterinary technician. Sedation was induced with isoflurane gas through a nose cone.
Surgical Procedure
Animals were randomly assigned to 3 different tenodesis groups: tendon-to-bone in the bicipital groove (metaphyseal, M); tendon-to-bone in the subpectoral region (diaphyseal, D); and soft tissue-to-soft tissue transfer to the conjoint tendon (T). A standard deltopectoral approach was used to expose the biceps tendon. The tendon was tagged with a 5-0 polypropylene suture and tenotomized at the level of the bicipital groove (zone 1). All wounds were irrigated and closed with 4-0 nylon suture.
For animals undergoing tendon-to-bone metaphyseal tenodesis, a 0.045-mm Kirschner wire was used to drill bicortically into the intertubercular sulcus. Wire positioning distal to the physeal plate was confirmed with fluoroscopy. A locking stitch of 5-0 polypropylene suture was run along the free edge of the tendon. The tendon was then passed through the bone tunnel in an anterior-to-posterior direction, and the limbs of the suture were tied around the lateral cortex.
The process was repeated for animals undergoing diaphyseal tenodesis; only the tenodesis location was different. The inferior border of the pectoralis major was identified, and a bicortical tunnel was made in the center of the diaphyseal bone. The tendon was then prepared and tenodesed to bone using the method already described.
In soft-tissue tenodesis, the conjoint tendon was identified and carefully dissected from surrounding tissues. The LHBT was then tenodesed to the attached conjoint tendon with interrupted simple stitches of 5-0 polypropylene suture.
The animals were allowed to bear weight on the operative limb immediately after surgery and without immobilization.
Specimen Harvest and Preparation
Four animals from each group were sacrificed at 6, 12, and 24 weeks. Harvested specimens were fixed in 10% neutral-buffered formalin solution. Bony specimens consisted of the upper half of the humerus and the tenodesed biceps tendon, and soft-tissue specimens consisted of the tenodesed LHBT-conjoint tendon complex. Bony specimens were decalcified in 10% ethylenediaminetetraacetic acid. All specimens were paraffin-embedded and sectioned at 7 microns.
Analysis of Cellularity
Sections were stained with hematoxylin-eosin. Overall cellularity at the tenodesis interface was quantified by averaging the nuclei count within 3 separate standardized ×20 magnification high power fields. Only nucleated cells were included in the cell count. Immunohistochemical staining with tenomodulin (Santa Cruz Laboratories, sc-49324) was performed to characterize the cell population at the interface. Deparaffinized sections underwent antigen retrieval with pronase for 30 minutes at 37°C and were incubated overnight with the anti-tenomodulin goat monoclonal antibody diluted to 1:200 in 1% phosphate-buffered saline. The prepared slides were then counterstained with methyl green. Specimens treated with tenomodulin were evaluated for presence or absence of a positive reaction at the tenodesis interface.
Analysis of Inflammation
Inflammation at the interface was evaluated with the CD68 macrophage marker (ABcam, ab31630). Deparaffinized sections underwent antigen retrieval with pronase for 30 minutes at 37°C and were incubated overnight with anti-CD68 mouse monoclonal antibodies diluted to 1:200 in 1% phosphate-buffered saline. The prepared slides were then counterstained with neutral red. Inflammation was quantified by averaging the number of reactive cells within 3 separate standardized ×20 magnification high power fields.
Statistical Analysis
Descriptive statistics were calculated for cell and macrophage counts for each group at every time point. Two-way analysis of variance was used to compare the cell and macrophage counts between groups at each time point as well as the count differences within each group between time points. P values were Bonferroni-corrected to account for the multiple comparisons between groups. P < .05 was used to signify statistical significance.
Results
All 36 animals survived to their designated harvest time without complications. Twelve specimens were successfully harvested at 6 weeks and another 12 at 24 weeks. At 12 weeks, tenodesis failure occurred in 1 animal in group D, leaving 11 specimens for analysis.
Cellularity
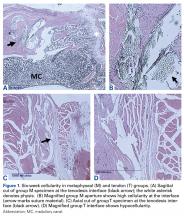
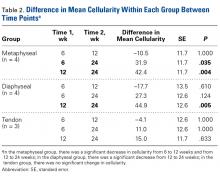
At the 6-week harvest, 1 group T specimen was sectioned incorrectly and discarded, leaving 3 specimens for evaluation. Descriptive statistics for each group and each time point are listed in Table 1A . There were no significant differences in mean cellularity between the bony tenodesis groups at any time point ( P = 1.000) ( Table 1B ). At 6 weeks, both group M ( P = .012) and group D ( P = .021) showed significantly more cellularity than group T ( Figure 1 ). The same was true for group M ( P = .002) and group D ( P = .001) at the 12-week harvest. There were no significant differences in cellularity between the 3 groups at 24 weeks.Within-group analysis revealed a trend of increasing cellularity at 12 weeks followed by a decrease at 24 weeks in all 3 groups ( Table 2 ).
Group M showed a significant overall decrease in cellularity from 6 to 24 weeks ( P = .035) and from 12 to 24 weeks ( P = .004). The overall decrease in cell count from 6 to 24 weeks was not significant in group D ( P = .124), whereas the decrease from 12 to 24 weeks was significant ( P = .005). There were no significant differences in cellularity for group T between any of the time points.Inflammatory Response
During specimen processing, 1 group D specimen was severely degraded after pronase treatment, leaving 3 specimens for evaluation. Descriptive statistics for each group are listed in Table 3A .
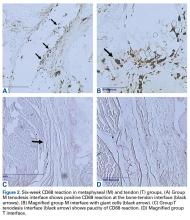
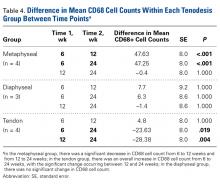
At 6 weeks, mean CD68 cell count was significantly higher in group M than in group D ( P = .011) and group T ( P < .001) ( Table 3B ). Likewise, CD68 count was significantly higher in group D than in group T ( P < .001). There were no differences in CD68 counts between the 2 bony tenodesis groups at 12 weeks ( P = .486) or 24 weeks ( P = .315). Both bony tenodesis groups, however, had persistently higher CD68 counts at 12 weeks when compared with group T (group M, P = .002; group D, P < .001). In these specimens, an inflammatory milieu characterized by a large accumulation of lymphocytes and giant cells was noted at the bone-tendon interface.
In comparison, inflammatory cells were scant at the tendon-tendon interface in group T specimens ( Figure 2 ). There were no differences in CD68 reaction between the 3 tenodesis groups at 24 weeks. In all cases of tendon-to-bone tenodesis, no recognizable formed tendon was seen within the bone tunnel. Rather, all tendon-to-bone tenodeses were characterized by dense connective tissue at the bone surface, surrounded by an accumulation of macrophages. Within-group analysis revealed a significant decrease in CD68 cells from 6 to 12 weeks and overall from 6 to 24 weeks in group M ( Table 4 ). There was no significant change in CD68 cell counts between any time points within group D ( P = 1.000) and between 6 and 12 weeks in group T ( P = 1.000). Interestingly, the CD68 counts in group T showed an overall increase from 6 to 24 weeks ( P = .019). The increase was even more significant from 12 to 24 weeks ( P = .004).Tissue-Specific Staining
At 6 weeks, antigen retrieval resulted in severe degradation of 2 group M specimens, 2 group D specimens, and 1 group T specimen. The most notable tenomodulin reaction occurred in group T at the 6- and 12-week harvests, with the 6-week group having the most robust reaction. There was scant reaction in this group at 24 weeks.
One group D specimen showed a positive reaction at 24 weeks. No reaction occurred in the remaining bony specimens across all time points ( Figure 3 ).Discussion
In this study, the healing response differed between bony and soft-tissue tenodesis techniques in a rat model. Tendon-to-bone tenodesis, both diaphyseal and metaphyseal, appeared to incite an inflammatory degenerative response, whereas tendon-to-tendon healing occurred in a more quiescent and perhaps even regenerative manner.
The early inflammatory response that occurred in the bony tenodesis groups is not unlike what occurs in fracture healing. 27 The reaction was even more robust at 12 weeks, signifying an ongoing inflammatory process. In this context, tendon degeneration may plausibly explain the consistent absence of mature tendon within the tunnels at all 3 time points. Some tendon degeneration may be explained by the vascular damage that occurred during surgery, but this damage was a constant factor in all 3 study groups. Interestingly, group M showed the highest early CD68 counts, consistent with this being the more biologically active region of bone. 28
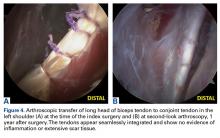
Group T had significantly lower cell and macrophage counts throughout the study period, possibly indicating improved healing—an observation supported by a study in which the impact of macrophage depletion on bone-tendon interface healing was evaluated. 29 The authors found that, in suppressing macrophage activity, the morphologic and biomechanical properties at the healing interface were significantly improved. 29 These findings are consistent with Dr. O’Brien’s anecdotal experience with patients who previously underwent the biceps transfer; on second-look arthroscopy, there was complete seamless integration of tendon and conjoint tendon ( Figure 4 ).
Studies have found that the inflammatory process is closely associated with pain, and pain syndromes such as fibromyalgia. 30,31 Persistent inflammation, as seen in our bony tenodesis group, could explain the recalcitrant anterior shoulder pain that often occurs in patients after bony tenodesis of the LHBT. 2,6,19,32
Studies have also suggested that osteoclasts at the bone-tendon interface—osteoclasts share a cell lineage with macrophages—may contribute to bone loss and tunnel widening. 33,34 Osteoclasts are expected at the bone tunnel, as fracture healing occurs at the bone-tendon interface. These osteoclasts could have contributed to the strong CD68 reaction in our bony tenodesis groups. However, CD68 historically has been described as the classic macrophage marker. 35 We specifically selected CD68 for this reason: Macrophages are the primary inflammatory cells involved in early healing and are key to the inflammatory process. 36
Results of the tenomodulin analysis suggested 2 different healing processes are occurring in the bony and tendon groups. Tenomodulin is a known tenocyte marker for developing and mature tendon in both rats and humans. 37,38 In our study, only group T had a positive tenomodulin reaction. Notably, the reaction occurred only at 6 and 12 weeks. This finding may indicate that a regenerative healing pattern becomes quiescent by 24 weeks. Indeed, it has been suggested that tenomodulin is a key regulator of tenocyte proliferation and tendon maturation. 39
The complete absence of tenomodulin reaction in our bony tenodesis groups in the setting of significant inflammation further supports our theory of tendon degeneration within the tunnel. One potential explanation for this finding may be that as the tendon heals to the surface of the bone, the intra-osseous tendon is no longer load-bearing and is resorbed by the body through an inflammatory response. This finding differs from those in previous studies, which have described viable tendon within the bone tunnel at all time points up to 26 weeks. 40 More recently, it has been suggested that callus formation at the external cortical tendon-bone interface is critical for healing and mechanical strength. 41,42 In addition, recent studies have found a predominantly fibroblastic healing process at the midtunnel, potentially leading to the formation of loose fibrovascular tissue at the tendon-bone interface. 43 These data, in concert with ours, call into question the rationale for performing intra-osseous tenodesis through bone tunnels.
Our study results, if confirmed in humans, will have significant clinical implications. If a similar effect can be confirmed in the human shoulder, one could argue that soft-tissue tenodesis may result in decreased postoperative shoulder pain. In addition, if tendon degeneration does occur within the intramedullary tunnel, surface fixation may be the better, safer alternative. Although older studies reported suboptimal strength with this type of fixation, 8,44 more recent studies have found surface fixation strength equivalent to screw fixation strength. 45,46 Such a shift in the treatment paradigm would obviate the need for violation of the humeral cortex, eliminating potential stress risers associated with screw fixation, 47 and effectively eliminating the risk of iatrogenic fracture. 48,49 It would be interesting to investigate what occurs histologically at the bone-tendon interface in surface fixation (ie, suture anchors). Would the inflammatory response at the surface be similar to the inflammatory intramedullary healing, or would it be similar to the quieter tendon-tendon healing? Answers to such questions have the potential to streamline the treatment algorithm for patients who require tenodesis.
Study Limitations
Our study had several limitations. First, as this was a basic science study using a rat model, its conclusions can only be extrapolated to humans. Second, given the nonspecific nature of the cellular analysis, we cannot draw any definitive conclusions about the cell population at the bone-tendon interface. For example, although tenomodulin is expressed by tenocytes, it is not an established specific marker for tenocytes and may be expressed by other fibroblastic cells. Still, our results provide insight into the local microenvironment and identify important differences between the tenodesis methods. Similarly, the complete absence of tendon within the bone tunnels suggests that an analysis of osteoclastic activity at the tenodesis interface may have been a valuable addition to the study. This finding, however, was unexpected, and we did not have the foresight to include it in our methods. A third limitation is that our fixation method essentially uses the suspension tenodesis method. This fixation method differs from the common fixation techniques used in the clinical setting. Testing of other fixation constructs would require a larger animal model. Furthermore, in suspension- type constructs, micromotion within the bone tunnel may independently elicit an inflammatory response. Inert suture was used in our fixation in order to reduce the risk of an iatrogenic inflammatory response. Last, it would have been valuable to perform a biomechanical analysis of the strength of each tenodesis construct. This was explored with our institution’s biomechanics team, but specimen size precluded successful analysis.
Conclusion
Our results indicated that, compared with tendon-to-tendon fixation, tendon-to-bone tenodesis produces a significantly greater inflammatory response at the tenodesis interface. An inflammatory milieu in the absence of tendon within the bony tunnel suggests intraosseous tendon degeneration. Tendon-to-tendon tenodesis, on the other hand, seems to limit the inflammatory response. In addition, a robust tenomodulin reaction in the early phases of tendon-to-tendon healing suggests regenerative healing. Our results showed a fundamental difference in the healing response between the 2 tenodesis methods. Further study is needed to evaluate the validity and applicability of our findings to the human patient population. Most important, our results underscore the need for more study to elucidate optimal tenodesis location and encourage orthopedic surgeons to reexamine current clinical practice patterns.