Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts or animal products. Infection of total knee or hip arthroplasty by Brucella species is a rare complication with only 18 cases reported in the English literature.1-12 We describe a case of an infected total hip replacement, its treatment, and 2-year follow-up and review the available literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
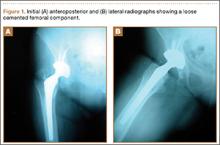
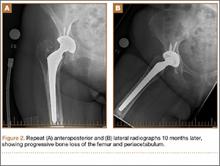
A 67-year-old Spanish-speaking woman, a native of Mexico, presented with a painful right total hip arthroplasty (THA) 2 years after implantation in Chihuahua, Mexico. The patient reported 1 year of increasing thigh pain with recent onset of start-up pain, and also mild groin pain. The patient reported an uneventful postoperative course without wound drainage and denied any history of fevers, chills, or night sweats after the procedure. Preoperative notes and radiographs were unavailable for review. Radiographic evaluation showed a hybrid construct with a well-fixed–appearing, uncemented acetabular component but a failed cemented femoral stem (Figures 1A, 1B). Although we discussed revision surgery, the patient elected not to proceed with surgery or to undergo evaluation to rule out infection. Nine months later, she returned with worsening pain and requested revision surgery; radiographs showed progressive bone loss around the cement mantle (Figures 2A, 2B).
Hematologic evaluation showed an erythrocyte sedimentation rate (ESR) of 54 mm/h (normal, 0-27 mm/h) and C-reactive protein (CRP) level of 0.24 mg/L (normal, <0.8). An aspiration of the hip with fluoroscopic guidance produced a small sample (0.2 mL) of yellow synovial fluid. There was not enough fluid for cell count, but fluid culture was negative.
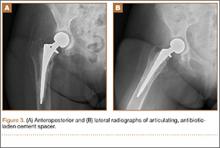
The patient was taken to the operating room for revision THA. Because of concern about progressive bone loss and elevated infectious indices, the administration of antibiotics was delayed until we obtained sufficient deep-tissue specimens. Before opening the capsule, we introduced a syringe into the joint and aspirated 10 mL of cloudy yellow synovial fluid that was sent for cell count. Additional findings at surgery included a grossly loose stem with a fragmented cement mantle surrounded by poor bone stock with anterior cortical bone loss and a loose acetabular component with pockets of cavitary bone loss. Frozen section showed up to 5 nucleated cells per high power field, and the cell count showed 1480 nucleated cells/µL (50% polymorphonuclear cells). The equivocal intraoperative findings (cell count and frozen section) and the loose femoral and acetabular components with significant bone loss were sufficiently concerning that we removed the components and placed a cement spacer rather than proceed with revision arthroplasty (Figures 3A, 3B). The surgeon, first assistant, and scrub technician wore body exhaust suits. We performed irrigation of the wound bed with pulse lavage.
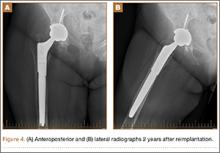
Intraoperative cultures (synovial fluid, joint capsule synovium, and femur pseudocapsule) were positive after 8 days and growing B abortus. Infectious disease consultants prescribed rifampin 300 mg twice daily and doxycycline 100 mg twice daily for 5 months. Follow-up ESR and CRP returned to normal range. A preoperative aspiration of the hip was negative as well. The patient returned to the operating room at 6 months for re-implantation using uncemented components; synovial fluid and tissue cultures taken at this time were negative. Two years after re-implantation, the patient is doing well without evidence of infection (Figures 4A, 4B). Additional follow-up will be required to monitor for infection and implant survival. Additional history taken from the patient after the culture results revealed that her development of hip pain was preceded by a febrile illness consistent with brucellosis.
Because of the nature of the procedure (irrigation and débridement using pulse lavage), we were concerned about aerosolization of Brucella bacteria and possible transmission to all staff present during the procedure. After consulting with the New Mexico Department of Health (NMDOH) and the Centers for Disease Control and Prevention (CDC), all surgical, anesthesia, and support personnel present in the operative suite and staff who cleaned the room after the procedure were treated prophylactically (rifampin 600 mg daily, doxycycline 100 mg twice daily for 3 weeks) to prevent development of brucellosis.13 All 15 operating room personnel who were exposed elected to proceed with antibiotic prophylaxis. In addition to prophylactic antibiotics, serial serologic testing for anti-Brucella antibodies was conducted at baseline and 2, 4, 6, and 24 weeks postexposure to monitor for the development of Brucella infection. There were no conversions to positive antibody status. No personnel complained of symptoms that would indicate development of brucellosis. At the recommendation of NMDOH and CDC, all staff in the operating room during and immediately after the re-implantation procedure wore properly fitting N-95 disposable respiratory masks (3M, St. Paul, Minnesota) to guard against the potential risk of further exposure.