News
European Commission expands denosumab indication
- Author:
- Mary Ellen Schneider
Following the FDA’s lead, the European Commission approves denosumab for prevention of skeletal-related events.
News
MRD may indicate relapse risk in AML
- Author:
- Mary Ellen Schneider
The presence of molecular minimal residual disease, despite complete remission, could be an independent predictor of future relapse risk.
Video

VIDEO: How to prepare PTCL patients for transplant
- Author:
- Mary Ellen Schneider
LA JOLLA, CALIF. – Dr. Steven Horwitz says the key to preparing patients for transplant is “good remission.”
News

FDA approves nilotinib for children with CML
- Author:
- Mary Ellen Schneider
The decision expands the drug’s indication to include first- and second-line use in children.
News

FDA approves new option in Hodgkin lymphoma treatment
- Author:
- Mary Ellen Schneider
The approval is based on results from the ECHELON-1 trial in previously untreated patients with stage III or IV classical Hodgkin lymphoma.
News
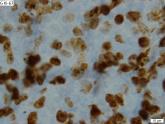
Time to rethink MCL treatment, trial design
- Author:
- Mary Ellen Schneider
Dr. Leonid Yavorkovsky of Kaiser Permanente San Jose Medical Center questions the traditional approach to treatment of class mantle cell lymphoma...
News
Consider steroid-induced hypertension when treating pediatric ALL
- Author:
- Mary Ellen Schneider
Infants and children with secondary diabetes mellitus were among those most likely to develop steroid-induced hypertension.
News
Hemophilia A treatment gains approval in Europe
- Author:
- Mary Ellen Schneider
Emicizumab is now approved in Europe and the United States for routine prophylaxis of bleeding episodes in people with factor VIII inhibitors.
News

FDA approves tests to screen for tickborne parasite in blood supply
- Author:
- Mary Ellen Schneider
The two new tests will screen whole blood and plasma samples.
News

Phase 2 study tests low-dose maintenance therapy for ALL
- Author:
- Mary Ellen Schneider
The trial is expected to enroll 40 adult ALL patients in complete remission with positive minimal residual disease.
News

Gene therapy for hemophilia A gets fast-track review
- Author:
- Mary Ellen Schneider
The FDA has granted breakthrough therapy designation to a gene therapy product aimed at hemophilia A.
News

FDA grants priority review for AML drug
- Author:
- Mary Ellen Schneider
The FDA is expected to make a decision in August 2018.
News
Three-drug combo delivers PFS for myeloma in OPTIMISMM trial
- Author:
- Mary Ellen Schneider
Celgene announced the phase 3 trial results, which investigators intend to present at future meetings.
News

Hemophilia A drug heads toward approval in Europe
- Author:
- Mary Ellen Schneider
The drug was approved by the U.S. Food and Drug Administration in November 2017.
News
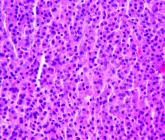
Real-world study makes case for continuous treatment in multiple myeloma
- Author:
- Mary Ellen Schneider
The study evaluated more than 600 adults with newly diagnosed multiple myeloma who relapsed.
