DENVER – Thirty-day transcatheter aortic valve replacement (TAVR) outcomes in real-world clinical practice using the Evolut R self-expanding valve were as good in patients treated for bicuspid disease as for tricuspid disease, according to a retrospective analysis of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) national registry.
This is encouraging news because at present only tricuspid aortic valve disease is an approved indication for TAVR. Bicuspid disease isn’t an approved indication because of a lack of supporting evidence regarding safety and efficacy. The new STS/ACC TVT registry data, which capture all commercial TAVR procedures done in the United States, lay the groundwork for an announced Medtronic-sponsored prospective study of Evolut Pro TAVR in patients with bicuspid disease aimed at winning an expanded indication for the device, which would open the door to on-label TAVR for patients with bicuspid disease, Jeffrey J. Popma, MD, explained at the Transcatheter Cardiovascular Therapeutics annual educational meeting (www.crf.org/tct).“I’ve always been insecure about whether we have the right technology to be able to treat bicuspid disease. This registry data is reassuring to me that we might. I think it may be time to do a prospective registry for low-surgical-risk patients with bicuspid disease and see if we can emulate these kinds of results,” said Dr. Popma, the director of interventional cardiology at Beth Israel Deaconess Medical Center and a professor of medicine at Harvard Medical School, both in Boston.
“I think that the one limitation to recruitment in our low-risk TAVR trial is patients with bicuspid disease. Probably 25%-30% of low-risk patients are bicuspid, so we can’t include them right now in our low-risk trial,” he added at the meeting sponsored by the Cardiovascular Research Foundation.
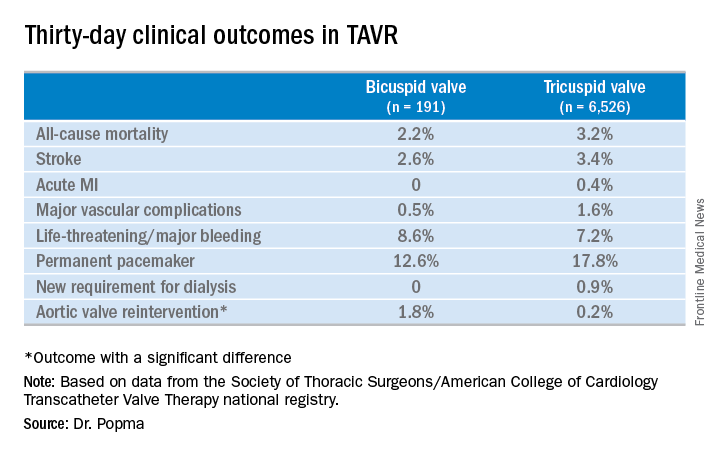
Even though TAVR for patients with bicuspid disease is off-label, operators do perform the procedure. All of these cases are captured in the STS/ACC TVT registry. Dr. Popma reported on 6,717 patients who underwent TAVR with placement of the Evolut R valve at 305 U.S. centers during 2014-2016. The purpose of this retrospective study was to compare 30-day outcomes in the 191 TAVR patients with native valve bicuspid disease with the outcomes in the 6,526 with tricuspid disease.
The two groups were evenly matched in terms of key baseline characteristics, including aortic valve mean gradient, severity of aortic, mitral, and tricuspid regurgitation, and comorbid conditions – with the exception of coronary artery disease, which was present in 48% of the bicuspid group versus 65% of those with tricuspid disease. Also, the bicuspid disease group was younger by an average of nearly 9 years, and their mean baseline left ventricular ejection fraction of 52.5% was lower than the LVEF of 55.5% seen in the tricuspid group.
Procedure time averaged 126 minutes in the bicuspid group and 116 in the tricuspid group. Femoral access was utilized in 87% of the bicuspid patients and in 92% of tricuspid patients. The device was implanted successfully in 97% of the bicuspid group and in 99% of the tricuspid group. More than one valve was required in 3.7% of the bicuspid disease group, a rate similar to that in the tricuspid group. Total hospital length of stay was roughly 6 days in both groups.
Rates of symptomatic improvement at 30 days were closely similar in the two groups. Preprocedurally, two-thirds of patients in both groups had a New York Heart Association class III; at 30 days, however, that was true for a mere 2.4% of the bicuspid patients and 10.3% of the tricuspid patients. By day 30, 52% of the bicuspid group and 48% of the tricuspid group were NYHA class I.
Also, 30-day rates of all-cause mortality, stroke, MI, major bleeding, and major vascular complications were similar in the two groups (see graphic). The only striking difference in 30-day clinical outcomes involved the need for aortic valve reintervention, which occurred in 1.8% of the bicuspid versus only 0.2% of tricuspid patients.No or only trace aortic regurgitation was present at 30 days in 62% of the bicuspid group and in 61% of the tricuspid group, while mild aortic regurgitation was noted in 31% and 33%, respectively.
Thirty-day mean aortic valve gradient improved to a similar extent in the two groups: from a baseline of 47.2 mm Hg to 9.4 mm Hg in the bicuspid group and from 42.9 mm Hg to 7.5 mm Hg in the tricuspid group.
Dr. Popma noted that an earlier analysis he carried out comparing outcomes of TAVR using the earlier-generation CoreValve in bicuspid versus tricuspid disease showed suboptimal rates of paravalvular regurgitation and an increased need for multiple valves in the bicuspid group.
“The lesson is ‘Thank God we’ve got new technology!’ because the new technology has made a big difference for us,” the cardiologist observed. “We think that the advancement in the technique and the advancement in the valves is going to give us fairly comparable outcomes with Evolut in bicuspid and tricuspid patients.”
Discussant Hasan Jilaihawi, MD, a codirector of transcatheter valve therapy at New York University, pronounced the short-term outcomes in patients with bicuspid aortic valve disease “better than I would have expected,” adding that he, too, thinks it’s time for a prospective registry study of the Evolut valve in such patients.
Dr. Popma’s study was supported by Medtronic. He reported having received research grants from Medtronic and other medical device companies.
SOURCE: Popma JJ. TCT 2017.