It included “triple therapy with renin-angiotensin system inhibitors, β-blockers, and mineralocorticoid receptor antagonists in 60% of patients, and at least double therapy in 90% of patients.” Also, Yancy said, 30% of the population had implantable devices, such as defibrillators and biventricular pacemakers.
Such patients with advanced, late-stage disease are common as the latest therapies for heart failure prolong their survival, notes Lynne W. Stevenson, MD, Vanderbilt University, Nashville, Tennessee, also as an invited discussant after Armstrong’s presentation.
“It’s a unique population with longer disease duration, more severe disease, and narrow options,” one in which personalized approaches are needed. Yet VICTORIA-like patients “have been actively excluded from all the trials that have shown benefit,” she said.
“VICTORIA finally addresses this population of decompensated patients,” she said, and seems to show that vericiguat may help some of them.
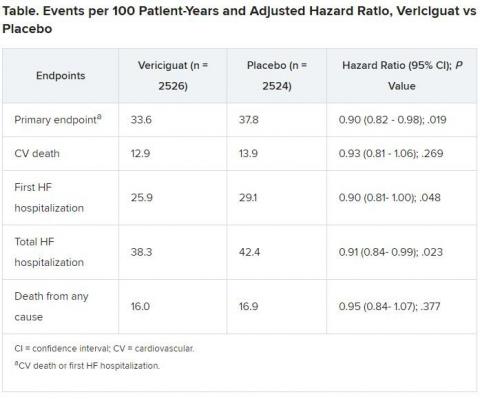
At the University of Glasgow, United Kingdom, John J.V. McMurray, MBChB, MD, agreed that the relative risk reduction was “small but significant,” but also that the control group’s event rate was “very high, reflecting the inclusion and exclusion criteria.”
As a result, McMurray told theheart.org | Medscape Cardiology, there was “quite a large absolute risk reduction and small number needed to treat. Also on the positive side: no significant excess of the adverse effects we might have been concerned about,” for example, hypotension.
Vericiguat, if ultimately approved in heart failure, “isn’t going to be first-line or widely used, but it is an additional asset,” he said. “Anything that helps in heart failure is valuable. There are always patients who can’t tolerate treatments, and always people who need more done.”
It’s appealing that the drug works by a long but unfruitfully explored mechanism that has little to do directly with the renin-angiotensin-aldosterone system.
Vericiguat is a soluble guanylate cyclase stimulator that boosts cyclic guanosine monophosphate activity along several pathways, potentiating the salutary pulmonary artery–vasodilating effects of nitric oxide. It improved natriuretic peptide levels in the preceding phase 2 SOCRATES-REDUCED study.
“This is not a me-too drug. It’s a new avenue for heart failure patients,” Armstrong said in an interview. It’s taken once daily, “was relatively easy to titrate up to the target dose, pretty well tolerated, and very safe. And remarkably, you don’t need to measure renal function.”
However, because the drug’s mechanism resides in the same neighborhood of biochemical pathways affected by chronic nitrates and by phosphodiesterase type 5 inhibitors, such as sildenafil and tadalafil, patients taking those drugs were excluded from VICTORIA. Acute nitrates were allowed, however.
Symptomatic hypotension occurred in less than 10% and syncope in 4% or less of both groups; neither difference between the two groups was significant. Anemia developed more often in patients receiving vericiguat (7.6%) than in the control group (5.7%).
“We think that on balance, vericiguat is a useful alternative option for patients. But certainly the only thing we can say at this point is it works in the high-risk population that we studied,” Armstrong said. “Whether it works in lower-risk populations and how it compares is speculation, of course.”
The drug’s cost, whatever it might be if approved, is another factor affecting how it would be used, noted several observers.
“We don’t know what the cost-effectiveness will be. It should be reasonable because the benefit was on hospitalization. That’s a costly outcome,” Yancy said.
McMurray was also hopeful. “If the treatment is well tolerated and reasonably priced, it may still be a valuable asset for at least a subset of patients.”
VICTORIA was supported by Merck Sharp & Dohme Corp and Bayer AG. Armstrong discloses receiving research grants from Merck, Bayer AG, Sanofi-Aventis, Boehringer Ingelheim, and CSL Ltd and consulting fees from Merck, Bayer AG, AstraZeneca, and Novartis. Y ancy has previously disclosed no relevant financial relationships. Stevenson has previously disclosed receiving research grants from Novartis, consulting or serving on an advisory board for Abbott and travel expenses or meals from Novartis and St Jude Medical. McMurray has previously disclosed nonfinancial support or other support from AstraZeneca, Bayer, Cardiorentis, Amgen, Oxford University/Bayer, Theracos, AbbVie, DalCor, Pfizer, Merck, Novartis, GlaxoSmithKline, Bristol-Myers Squibb, and Vifor-Fresenius.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Presented March 28, 2020. Session 402-08.
N Engl J Med. Published online March 28, 2020. Full text; Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.