A 10-year follow-up analysis based on one of cardiology’s most influential trials has shed further light on one of its key issues: how to sharpen selection of patients most likely to benefit from a primary prevention implantable cardioverter-defibrillator (ICD).
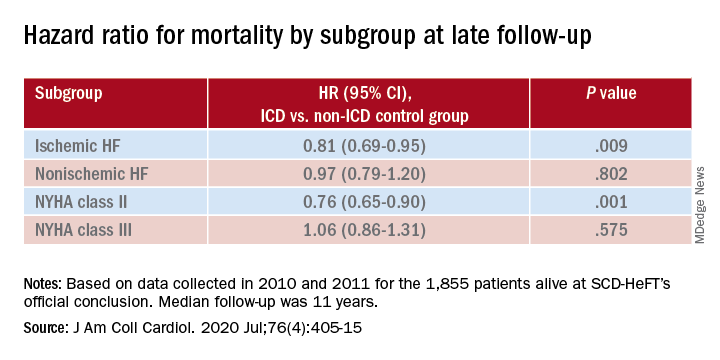
In a new report from SCD-HeFT, the survival advantage in patients with heart failure seen 5 years after receiving ICDs, compared with a non-ICD control group, narrowed a bit but remained significant after an additional 5 years. But not all patients with devices shared in that long-term ICD benefit. Patients with either ischemic disease or nonischemic cardiomyopathy (NICM) with devices showed a similar mortality risk reduction in the trial’s previously reported 5-year outcomes. That advantage, compared with non-ICD control patients, persisted throughout the subsequent 5 years for ischemic patients but tapered to nil for those with NICM.
The NICM patients “had what appears to be some accrual of benefit maybe out to about 6 years, and then the curves appear to come together where there’s no apparent further benefit after 6 years,” Jeanne E. Poole, MD, of the University of Washington, Seattle, said in an interview.
In both the 10-year analysis and the earlier results, ICD survival gains went preferentially to patients who enrolled with New York Heart Association (NYHA) functional class II symptoms. Patients who entered in NYHA class III “didn’t appear to have any benefit whatsoever” in either period, Dr. Poole said.
“The simple message is that the same groups of patients that benefited strongly from the ICD in the original SCD-HeFT – the NYHA class 2 patients and those with ischemic cardiomyopathy – were really the ones who benefited the greatest over the long term,” she said.
Dr. Poole is lead author on the SCD-HeFT 10-year analysis, which was published in the July 28 issue of the Journal of the American College of Cardiology.
Why the ICD survival effect disappeared midway in patients with NICM “is hard to sort out,” she said. Many in the control group were offered such devices after the trial concluded. Among those, it’s possible that disproportionately more control patients with NICM, compared with patients with ischemic disease, were fitted with ICDs that were also cardiac resynchronization therapy (CRT) devices, Dr. Poole and her colleagues speculated. That could have shifted their late outcomes to be more in line with patients who had received ICDs when the trial started.
Or “it is possible that the intermediate-term benefit of ICD therapy in NICM is overwhelmed by nonarrhythmic death in extended follow-up” given that ICDs prolong survival only by preventing arrhythmic death, noted an editorial accompanying the new SCD-HeFT publication.
Another possibility: Because NICM is a heterogeneous disorder with many potential causes, perhaps “the absence of long-term mortality benefit among SCD-HeFT participants with NICM was due to an unintended but preferential enrollment of subtypes at relatively lower risk for arrhythmic death in the longer term,” proposed Eric C. Stecker, MD, MPH, Oregon Health & Science University, Portland, and coauthors in their editorial.
“What are the take-away messages from the current analysis by Poole et al?” they asked. “These findings strongly support the clinical efficacy and cost-effectiveness of ICD therapy for the majority of patients with severe but mildly symptomatic ischemic cardiomyopathy who do not have an excessive comorbidity burden.”
But “the implications for patients with NICM are less clear,” they wrote. “Given evidence for intermediate-term benefit and the limitations inherent to assessing longer-term benefit, we do not believe it is appropriate to walk back guideline recommendations regarding ICD implantation for NICM patients.”
The findings in nonischemic patients invite comparison with the randomized DANISH trial, which entered only patients with NICM and, over more than 5 years, saw no primary-prevention ICD advantage for the end point of all-cause mortality.
But patients who received ICDs showed a reduction in arrhythmic death, a secondary end point. And mortality in the trial showed a significant interaction with patient age; survival went up sharply with ICDs for those younger than 60 years.
Also in DANISH, “the ICD treatment effect appears to vary over time, with an earlier phase showing possible survival benefit and a later phase showing attenuation of that benefit,” similar to what was seen long-term in SCD-HeFT, in which the interaction between mortality and time since implantation was significant at P = .0015, observe Dr. Poole and colleagues.
However, Dr. Poole cautioned when interviewed, patient management in DANISH, conducted exclusively in Denmark, may not have been representative of the rest of the world, complicating comparisons with other studies. For example, nearly 60% of all patients in DANISH had defibrillating CRT devices. Virtually everyone was on ACE inhibitors or angiotensin-receptor blockers, and almost 60% were taking aldosterone inhibitors.
“DANISH is an unusually high bar and probably does not reflect all patients with heart failure, and certainly does not reflect patients in the United States in terms of those high levels of guideline-directed medical therapy,” Dr. Poole said. The message from DANISH, she said, seems to be that patients with NICM who are definitely on goal-directed heart failure medications with CRT devices “probably don’t have a meaningful benefit from an ICD, on total mortality, because their sudden death rates are simply so low.”
SCD-HeFT had originally assigned 2,521 patients with heart failure of NYHA class II or III and an left ventricular ejection fraction of less than 35% to receive an ICD, amiodarone without an ICD, or an amiodarone placebo and no ICD; patients in the latter cohorts made up the non-ICD control group.
Those who received an ICD, compared with the non-ICD control patients, showed a 23% drop in all-cause mortality over a median of 45.5 months ending on October 31, 2003, Dr. Poole and colleagues noted in their current report. The trial’s primary results were unveiled 2005.
The current analysis, based on data collected in 2010 and 2011, followed the 1,855 patients alive at the trial’s official conclusion and combined outcomes before and after that time for a median follow-up of 11 years, Dr. Poole and colleagues reported.
In the ICD group, the overall hazard ratio for mortality by intention-to-treat was 0.87 (95% confidence interval, 0.76-0.98; P = .028), compared with the non-ICD control group.
In their report, Poole and associates clarified one of the foremost potential confounders in the current analysis: device implantations after the trial in patients who had been in the non-ICD groups. From partial clinical data collected after the trial, they wrote, the estimated rate of subsequent ICD implantation in non-ICD control patients was about 55%. Such a low number is consistent with clinical practice in the United States, where “a surprisingly low number of patients who are eligible actually end up getting devices,” Dr. Poole said.
Subsequent ICD use in the former non-ICD control patients presumably boosted their survival over the long term, narrowing the gap between their all-cause mortality and that of the original ICD patients, Dr. Poole observed. Despite that, the ICD-group’s late survival advantage remained significant.
SCD-HeFT was sponsored by Medtronic, Wyeth Pharmaceuticals, and the National Heart, Lung, and Blood Institute. The current analysis was partially supported by a grant from St. Jude Medical. Dr. Poole disclosed receiving research support from Medtronic, Biotronik, AtriCure, and Kestra; serving as a speaker for Boston Scientific, Medtronic, and MediaSphere Medical and on an advisory board for Boston Scientific; serving on a committee for Medtronic and on a data and safety monitoring board for EBR Systems; and receiving royalties from Elsevier and compensation from the Heart Rhythm Society for serving as editor in chief for the Heart Rhythm O2 journal. Disclosures for the other authors are in the report. Dr. Stecker and coauthors disclosed that they have no relevant relationships.
A version of this article originally appeared on Medscape.com.