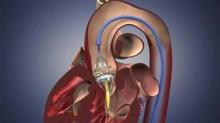
CHICAGO – Approval of the first transcatheter aortic valve will transform heart valve repair in the United States and prompt a range of inevitable clinical quandaries, experts suggest.
The Edwards SAPIEN transcatheter valve was approved Nov. 2 for inoperable patients with severe tricuspid aortic stenosis, but already transcatheter aortic valve implantation (TAVI) is being eyed for congenital bicuspid aortic valves and as an alternative strategy for patients with degenerating bioprostheses, Dr. Howard Herrmann said at the 2011 Heart Valve Summit.
The feasibility of TAVI in symptomatic, severe bicuspid aortic valve was recently demonstrated in 11 patients, aged 52-90 years, with one case of malpositioning and late migration at 63 days, but no more aortic insufficiency than in tri-leaflet aortic valves (J. Am. Coll. Cardiol. Intv. 2010;3:1122-25).
"At the moment, young patients are not the ideal candidates for PARTNER or TAVI valves, but by the same token, we do see bicuspid valves in the 80-plus group and I think there are still some unknowns about how to use this device in that setting," said Dr. Herrmann, director of the interventional cardiology program and cardiac catheterization laboratories at the University of Pennsylvania Medical Center in Philadelphia.
Unknowns include the risk of malposition, long-term outcome, aortic insufficiency and the effect of calcification, and anatomy such as bulky leaflets and dilated root.
Successes with "valve-in-valve" implantations have been mostly transapical to date, Dr. Herrmann said at the meeting, cosponsored by the American Association for Thoracic Surgery and American College of Cardiology Foundation. Use of the transapical approach resulted in a success rate of 96% among 24 high-risk patients with failed bioprostheses (Circulation 2010;121;1848-57). The stroke rate was 4% and there were no postprocedure embolizations.
"This has clearly already altered the discussion we have with patients," Dr. Herrmann said. "I see patients in my office all the time who are 50 to 55 [years old] who want a bioprosthetic valve because they don’t want to be on Coumadin, but expect us to figure out how to put a transcatheter valve in them in 15 years.
"There are certainly unknowns, but it’s out there in the community and something we have to think about, so that we can give intelligent answers to these patients."
The overall TAVI landscape is expected to change even more in light of the European approval in recent weeks of two second-generation transapical TAVI devices – JenaValve Technology’s JenaValve both available in three sizes covering aortic annulus diameters from 21 mm to 27 mm.
In November, Medtronic also announced that its CoreValve system, currently limited to investigational use in the United States, has gained approval in Europe as the first transcatheter valve to be delivered using direct aortic access.
Dr. David H. Adams, coprincipal investigator of the CoreValve-Medtronic U.S. Pivotal trial and professor and chair of cardiothoracic surgery at Mount Sinai Medical Center in New York City, said alternative arterial access is being evaluated in both the ongoing CoreValve Extreme Risk and High Risk studies, which has implications because patients treated with apical access in the PARTNER (Placement of AoRTic TraNcathetER Valve) trial had more adverse events.
The use of smaller devices like Medtronic’s 18-French CoreValve prosthesis also has implications for clinical practice because the smaller system can access small femoral arteries and more diseased femoral vessels, he said. Among 126 patients with calcific aortic stenosis, including 54 patients with moderate surgical risk, the overall success rate with the 18-F CoreValve device was 83% and 30-day all-cause mortality 15% (J. Am. Coll. Cardiol. 2011;57:1650-7). All-cause mortality at 2 years was significantly improved among moderate-risk patients, but this was attributed to an increased risk of noncardiac mortality among high-risk patients.
The approval of the Edwards SAPIEN and CoreValve in Europe in 2007 has led to what some describe as a "runaway train" in Europe, where patients and physicians are voting with their feet. TAVI procedures made up just 1.2% of valve procedures in 2007, but are expected to exceed 30% in the first half of 2011 in some countries, Dr. Herrmann said. Symetis, which is launching a 150-patient pivotal trial of its new device in the U.S., estimates that the TAVI market will exceed $2 billion by 2014.
This explosive growth in Europe has been driven by reimbursement, and has resulted in a drift in TAVI usage among lower-risk patients. The mean logistic EuroScore now in some series has fallen below 15% or comparable to a Society of Thoracic Surgery (STS) score of 5%, Dr. Herrmann said.