News

The permanent implantable cardiac heart pump was developed in the mid-20th century as an outgrowth of the success of heart-lung machines –which provided systemic support during cardiac arrest – required for valve replacement and later coronary bypass surgery.
The challenge to build a totally implantable heart has been the "holy grail" for almost half a century and occurred long before heart failure therapy was high on the agenda of cardiologists. It emerged as a result of the experimental perseverance and genius of Dr. Willem Kolff, who in 1957 was able to totally support dogs using an artificial heart device. Other surgeons, working in separate laboratories – including Dr. Adrian Kantrowitz, Dr. Denton Cooley, and Dr. Robert Jarvik – provided additional research support for the ultimate creation of the artificial heart. However, it wasn’t until 15 years later, in 1982, that Dr. Kolff captured the attention of the medical and lay press by supporting a Seattle dentist suffering from severe heart failure, Dr. Barney Clark, for 112 days with the heart that he and Dr. Jarvik had developed.
Since then, research on the totally implantable heart led to the approval in 2004 the SynCardia temporary Total Artificial Heart as a bridge to transplantation in patients with biventricular failure. In 2001, the first AbioCor totally implantable pump with an external power source was implanted. Initially approved by the FDA as a bridge to transplant for patients with biventricular failure, more recently it has been approved for patients with end stage heart failure as destination therapy.
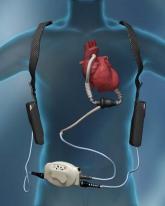
As work went forward on the totally implantable heart, left ventricular assist devices (LVAD) were also being developed. The pharmacologic support of end stage left ventricular failure with vasodilators and inotropic agents has provided modest temporary benefit; but it has become obvious that we have reached a therapeutic wall with very few new medical options on the horizon. LVADs appeared to be our current best hope of providing additional short- and long-term support for the failing left ventricle.
Dr. E. Stanley Crawford and Dr. Domingo Liotta performed the first LVAD implant in 1966 in a patient who had cardiac arrest after surgery. Since then, there have been a variety of LVADs developed that were initially pulsatile, but now are more commonly continuous flow. Both types of devices are externally powered via drive lines and able to achieve flows up to 10 L/min and are interposed between a left ventricular apical conduit and an ascending aorta conduit. The initial LVADs were pulsatile devices based on the presumption that pulsatile flow was important for systemic perfusion and normal physiology. However, continuous-flow LVAD has proven to be quite compatible with normal organ function and perfusion, and shown better durability and lower mortality and morbidity compared to the pulsatile flow devices. (J. Am. Coll. Cardiol. 2011;57:1890-8).
In addition, as noted in "The Lead," LVADs have shown superiority over medical therapy in patients with advanced heart failure as destination therapy, and the 1-year mortality with continuous flow LVADs now approximates the experience with the 1-year mortality of patients receiving a heart transplant.
The expanded use of LVADs from creating a bridge for transplantation to destination therapy has opened an entirely new opportunity for the use of LVADs in the treatment of acute, but most importantly, chronic heart failure. The limitation of heart transplantation as a function of donor availability together with the limitation of medical therapy for heart failure patients has generated increased interest in LVADs for chronic therapy in patients with end-stage heart failure. The observation that in some patients, particularly those with reversible heart failure like myocarditis, the heart may actually recover during LVAD therapy and allow for its removal, provides a window into future clinical applications (N. Engl. J. Med. 2006:355;1873-84)
The potential for further miniaturization of these devices and the potential for total implantability also open new horizons for LVAD therapy. Total implantability hinges on the ability to apply technology of transcutaneous power source that is already available in a number of electronic implantable devices, including the total heart implant. The resolution of these technical issues will allow for further expansion of the clinical indications for LVAD therapy.
Dr. Goldstein, medical editor of Cardiology News, is a professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.

