CHICAGO – Treatment with a dipeptidyl peptidase-4 inhibitor in real-world community practice doesn’t increase the risk of cardiovascular events in type 2 diabetic patients; in fact, the opposite was true in a very large population-based cohort study.
The study, hailed by audience members as "a tour de force" and "a really excellent study," involved 39,769 type 2 diabetes patients who initiated treatment with a dipeptidyl peptidase-4 inhibitor (DPP-4i), either as monotherapy or, far more commonly, on top of metformin, and another 39,769 patients with type 2 diabetes on metformin who initiated combination therapy with a non–DPP-4i hypoglycemic agent. The two groups were closely matched using propensity scores that incorporated 50 variables, including comorbid conditions, medications, hemoglobin A1c, and health care utilization.
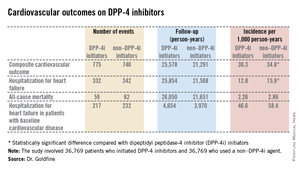
The primary endpoint was a composite cardiovascular outcome comprising acute MI, stroke, coronary revascularization, and hospitalization for heart failure. The rate was 30.3 per 1,000 person-years in patients after initiating DPP-4i therapy and 34.76 per 1,000 in controls who initiated treatment with a non–DPP-4i, for a statistically significant 13% relative risk reduction, Dr. Allison B. Goldfine reported at a joint meeting of the International Congress of Endocrinology and the Endocrine Society.
Also noteworthy was the finding that patients who initiated treatment with a DPP-4i had a 19% lower subsequent risk of hospitalization for heart failure than did those who initiated therapy with another glucose-lowering agent (see chart). This statistically significant reduction in heart failure hospitalization was particularly reassuring. Earlier, the roughly 16,500-subject, randomized, SAVOR-TIMI 53 trial showed a significant 27% increased risk of hospitalization for heart failure in patients assigned to saxagliptin, compared with placebo-treated controls (N. Engl. J. Med. 2013;369:1317-26).
Moreover, the EXAMINE study, another randomized trial, albeit smaller, reported a nonsignificant 19% increased relative risk of hospitalization for heart failure, compared with placebo (N. Engl. J. Med. 2013;369:1327-35). These findings raised a red flag at the Food and Drug Administration, which sought additional information.
Both SAVOR-TIMI 53 and EXAMINE involved type 2 diabetes patients with known cardiovascular disease at enrollment. In the new population-based cohort study, 7,293 of the 39,769 patient pairs had baseline cardiovascular disease. Reassuringly, those with baseline cardiovascular disease had a 12% relative risk reduction for the composite cardiovascular endpoint and a 16% lower risk of hospitalization for heart failure after going on a DPP-4i, compared with controls who initiated treatment with a non–DPP-4i. Both risk reductions barely missed achieving statistical significance because of insufficient patient numbers, said Dr. Goldfine, head of the section of clinical, behavioral, and outcomes research at the Joslin Diabetes Center, Boston, and an endocrinologist at Harvard University.
To investigate the additional possibility that going on DPP-4i therapy might somehow predispose to mild heart failure not severe enough to result in hospitalization, she and her coinvestigators looked at new use of loop diuretics among matched patient pairs not on that medication class at baseline. The risk of getting a new prescription for a loop diuretic during follow-up turned out to be lower by a statistically significant 26% in DPP-4i users, compared with nonusers.
The study utilized the UnitedHealthcare insurance claims database for 2005-2012. From an initial population of 4.35 million patients with type 2 diabetes, Dr. Goldfine and her coworkers utilized propensity scores to whittle down to a study population of just less than 40,000 very closely matched patient pairs.
The great majority of DPP-4i users in the study were on sitagliptin (Januvia), the first-in-class agent to reach the market.
The study was funded by the National Institutes of Health. Dr. Goldfine reported serving as an investigator on studies funded by Daiichi Sankyo, NovoNordisk, and other companies.