Takotsubo cardiomyopathy (TTC) is characterized by transient wall motion abnormalities of the left ventricle (LV), resulting in apical ballooning. Despite sporadic reports noting right ventricular (RV) involvement, research to date has mainly focused on LV pathology. However, Elesber and colleagues, the first group to systematically evaluate RV involvement in TTC, found RV dysfunction in eight of 25 patients.1
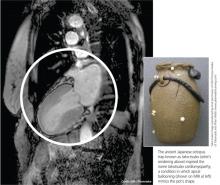
The condition is known by many different names: stress-induced cardiomyopathy, broken heart syndrome, and ampulla cardiomyopathy. The term takotsubo cardiomyopathy is derived from the appearance of the mid-ventricle and apex of the heart on echocardiography or catheterization during systole; this apical ballooning resembles a spherical bottle with a narrow neck, not unlike the ancient Japanese octopus trap called tako-tsubo (see image). First described in Japanese articles in 1991 by Dote and colleagues, TTC is not typically associated with coronary artery stenosis on angiography.2
TTC is diagnosed in approximately 1% to 2% of patients who present with signs and symptoms similar to those of acute myocardial infarction (AMI).3 The majority of affected patients are postmenopausal women, with two studies showing an 89% to 90% female predominance and mean age at presentation ranging from 58 to 77 and 58 to 75 years, respectively.4,5
The pathogenesis of TTC has been postulated to include multivessel epicardial spasm, catecholamine-mediated myocardial damage, microvascular coronary spasm or dysfunction, and neurogenic myocardial stunning.6 TTC is often not recognized on initial presentation, as it mimics AMI with ST elevation. Providers should maintain a high clinical suspicion for this transient clinical condition, which is increasingly recognized in various populations.4
Etiology and Pathophysiology
The etiology of TTC remains unclear, but a physiologic or emotional stressor usually precedes the onset of symptoms.7 It is hypothesized that a catecholamine surge in response to a stressor causes myocardial stunning through an uncertain mechanism.
Catecholamine-induced myocardial stunning due to various stressors has been documented through measurement of plasma levels of these hormones in more than 70% of patients with TTC.3 Myocardial scintillography with 123I-metaiodobenzylguanidine (MIBG) in these patients demonstrated a decreased uptake of a radiotracer in several segments of the LV, emphasizing a severe adrenalin secretion production due to stress. Considerable individual differences in MIBG uptake in patients with TTC may reflect variable responses to adrenergic stimulation, due to differences in genetic inheritance of adrenalin synthesis, functions, storage, and elimination.8
Additionally, Lyon and colleagues demonstrated a higher density of beta-adrenergic receptors in the apical region in these patients.9 They hypothesized that excessive levels of circulating catecholamines significantly alter the apical heart muscle cells, decreasing the force or energy of the muscle contraction. Conversely, Dandel and colleagues suggest that the akinetic appearance of this region can be related to high systolic apical circumferential wall stress.10
A recent literature review of 42 articles reported a possible link between TTC and drugs that overstimulate the sympathetic system.11 Consequently, clinicians should maintain a high suspicion for drug-induced TTC in patients who present with symptoms consistent with TTC but who have not experienced particularly stressful events immediately prior to onset.
The reason TTC predominately affects postmenopausal women remains unclear, but a link with reduced levels of estrogen and effects on the microvascular system has been proposed.5 One study reports a patient with TTC who carried a mutation of gene FMR1 (alleles with sizes between 40 and 55 triplet permutations); the researchers recommend further study of the role of cardiac genes in the acute phase of TTC.12 A familial apical ballooning syndrome was reported in a mother and daughter in another study, suggesting that certain women may be genetically predisposed to TTC; this may explain why only a minority of postmenopausal women are susceptible.13 However, there is no known association with single nucleotide polymorphisms for adrenergic receptors (based on a study that compared women with TTC to a control group).14
Patient Presentation
Chest pain is one of the most concerning symptoms for patients and primary care providers. The priority for a patient presenting with chest pain is to exclude catastrophic or life-threatening causes. Therefore, primary care providers are encouraged to refer patients to the nearest emergency department for appropriate diagnostic evaluation.
Precipitating events are typically severe emotional or physiologic stressors, but the absence of such a stressor does not exclude the diagnosis. Examples of emotional stressors include learning of the death of a loved one, a personal financial blow, legal problems, natural disasters, and motor vehicle collisions.4 Physiologic stressors, such as a severe medical illness, worsening chronic disease, or a noncardiac surgical procedure, can also trigger an episode of TTC.15
Patients present with chest pain and dyspnea that resemble the symptoms of AMI.4 Most patients will report chest pain at rest, although some may have dyspnea as their sole presenting complaint. They may also report palpitations, nausea, and vomiting. Patients who present with TTC typically have few traditional cardiac risk factors, such as hypertension, hyperlipidemia, diabetes, smoking, or a family history of cardiovascular disease.3