A variety of findings have been reported in the history and physical exam of most patients who present with CD.The most prevalent signs and symptoms are abdominal pain, frequent loose stools, weight loss, joint pain, and weakness.8,11,16 Unlike the pediatric patient with the classic triad of symptoms, adults usually experience more generalized GI manifestations, such as irritable bowel syndrome (IBS), abdominal pain, or acid reflux.10
Many patients have no GI symptoms but may present solely with fatigue, arthralgias, or myalgia.20 In fact, more than 50% of adults with CD present with atypical or extraintestinal disorders, such as anemia, infertility, osteoporosis, neurologic problems, or other autoimmune disorders.8,16,23,24 It is important for clinicians to note that atypical is somewhat typical in the older patient who presents with CD.
Patients with asymptomatic or silent CD, (see “Classification and Pathology,” below) lack both classic and atypical symptoms but still have villous atrophy, usually discovered during endoscopy being conducted for other reasons.8 Because of its predominantly atypical presentations, CD is considered a multisystem endocrine condition rather than one that is mainly gastrointestinal.8,16,25,26
CLASSIFICATION AND PATHOLOGY
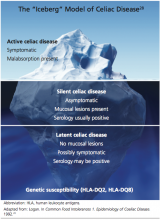
Though frequently a silent disorder, CD typically progresses through four stages: classical, atypical, latent, and silent. Clinicians should strive to become fully aware of each stage and its implications.8,26,27
The classical form is primarily diagnosed in children ages 6 to 18 months. It is characterized by villous atrophy and typical symptoms of intestinal malabsorption.8
The patient with atypical CD has minor intestinal symptoms, but architectural abnormalities can be found in the mucosa of the small intestine. This patient is likely to present with various extraintestinal disorders, including osteoporosis, anemia, infertility, and neuropathies.7,8
In the latent form of CD, the HLA-DQ2 and/or -DQ8 genetic markers are present. Serology for CD may be positive, but the intestinal mucosa may be normal. The patient may or may not be experiencing extraintestinal symptoms. In patients with latent CD, the gluten-associated changes appear later in life.8 The precise trigger for late activation of the disease, though apparently linked to genetics and gluten exposure, remains elusive.20,24
The silent form of CD is marked by mucosal abnormalities in the small intestine and usually by positive CD serology, but it is asymptomatic. The iceberg theory of celiac disease28 (see figure28) has been proposed to explain CD’s hidden manifestations over time.
In patients with atypical, latent, or silent CD, the condition is sometimes detected incidentally during screening of at-risk groups or by endoscopy performed for other reasons.8 Most of these patients respond well to GFD therapy, noting both physical and psychological improvement—suggesting that these patients, even though asymptomatic and seemingly healthy, may have been experiencing minor manifestations of undiagnosed CD for many years: decreased appetite, fatigue, and even behavioral abnormalities.1,8
Histopathologic analysis of abnormalities found on biopsy of the small intestine relies on the four-stage Marsh classification29:
• Marsh 0: normal mucosa
• Marsh I: intraepithelial lymphocytosis
• Marsh II: intraepithelial lymphocytosis with crypt hyperplasia
• Marsh III: intraepithelial lymphocytosis with crypt hyperplasia and villous atrophy.8,29 Modifications to this classification have been made by Oberhuber30,31 to denote the degree of villous flattening32 (ie, IIIa, IIIb, IIIc).
Villous atrophy of the mucosa has long been considered the hallmark of CD, and its detection, according to the American Gastroenterological Association,2,26 remains the gold standard in confirming a diagnosis of CD.4,16,26 However, early screening (ie, serologic testing for tTG and EMA) is the necessary initial step in ensuring diagnostic accuracy, as other conditions can cause villous atrophy, and latent CD can coexist with normal intestinal mucosa.10
Avoiding Diagnostic Delays
Because of the broad spectrum of unrelated GI signs in all ages and the subtle presentation in adults, diagnosis of CD in this patient population is frequently delayed for estimated periods ranging from five to 11 years.4,11,23,33
Improving clinician awareness of the manifestations of CD is essential34; too frequently, the common symptoms of probable CD are treated as individual idiopathic disorders by both PCPs and secondary specialists, who prescribe proton pump inhibitors, antihistamines, cathartics, and/or antimotility drugs for years without ruling out a common, easily identified genetic disease. Even though the prevalence of CD has recently been shown to have increased more than fourfold since 1950,35 serologic testing for CD is not widely implemented by PCPs.4,11,20
Specialists, too, may be slow to recognize this treatable autoimmune disorder. In a recent nationwide study, it was found that gastroenterologists performed a small-bowel biopsy in less than 10% of their patients who underwent esophagogastroduodenoscopy (EGD) for likely symptoms of CD.36 Relying solely on clinical expertise and visual recognition of intestinal abnormalities can delay diagnosis for years.4,36 Many patients may never be given a correct diagnosis of CD.