CLINICAL MANIFESTATIONS
Due to the widespread use of cervical cytology, many cases of CIN and cervical cancer are diagnosed well before symptoms develop. In the early stages of cervical cancer, most women are asymptomatic, although some women may present with a watery, blood-tinged, or malodorous vaginal discharge.7 Any type of abnormal bleeding, whether it is between menstrual cycles, postcoital, or postmenopause, warrants an evaluation for pathologic processes, including but not limited to cervical cancer. Patients with late-stage cervical cancer may present with complaints of pelvic pain and painful intercourse due to the enlargement of a cervical mass. Vaginal discharge may change in consistency from watery to purulent and become foul-smelling due to cervical tissue necrosis.7,9,22 In some cases of advanced invasive cervical cancer, patients will experience hematuria or rectal bleeding secondary to tumor invasion through the bladder or rectal wall.9 Other nonspecific signs and symptoms of cervical cancer include unexplained weight loss accompanied by nausea or vomiting, as well as loss of appetite.7,9
SCREENING AND PREVENTION
Widespread use of the Pap test since the 1950s has led to marked reductions in the incidence of cervical cancer. The Pap test enables clinicians to screen for and detect CIN. More recently, liquid-based cytology has been utilized for the same purpose. The Pap smear involves placing the sample cells directly onto a microscopic slide. With liquid-based cytology, the sample is placed into a vial,2,7 where a portion is processed in preservative liquid under light microscopy for review by a cytologist.7 The remainder of the cell sample can be tested for HPV and STIs.
DNA testing for high-risk HPV subtypes is available and has been incorporated into the cervical cancer screening guidelines. The guidelines recommend HPV DNA testing as a co-test to be performed with cytology in women older than 30.2,24 The recommendation for HPV DNA testing in this age-group reflects greater concern that older women’s immune systems will not clear the virus, leaving these women at higher risk for developing CIN and cancer.25,26 HPV-16 and HPV-18 genotyping is available and is recommended in women 30 or older whose co-testing reveals normal cytology in the presence of a high-risk HPV subtype.27
Additionally, HPV DNA testing is used to triage cytology that reveals atypical squamous cells of undetermined significance. HPV DNA testing is not performed routinely in younger women because of the high likelihood that their immune systems will clear the virus. Genetic testing for the 3q26 gene amplification is available but has not yet been incorporated into the screening guidelines.27
Screening Guidelines
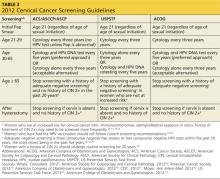
In September 2012, the American Cancer Society, American Society for Colposcopy and Cervical Pathology (ASCCP), and the American Society for Clinical Pathology developed a new set of cervical cancer screening guidelines.28 The US Preventive Services Task Force (USPSTF) and American College of Obstetricians and Gynecologists (ACOG) have also created standard recommendations for cervical cancer screening (see Table 32,28-34). Although ACOG’s standards have varied from those of other organizations in the past, their 2012 guidelines now align closely.
All these organizations recommend that cervical screening begin at age 21 for all women, regardless of the age of sexual initiation.31 Many young women who acquire an HPV infection will clear the infection within the first one to two years. Eliminating screening of women younger than 21 will help to avoid unnecessary biopsies and invasive treatment.29-32
All the guidelines include general recommendations for screening; however, each organization has made exceptions for patients at higher risk for developing cervical cancer.2,28-34 ASCCP has also developed algorithms for management of the multitude of abnormal cervical cancer screening results. These algorithms are available through the ASCCP Web site (www.asccp.org).
Vaccinations
HPV vaccines developed in recent years are now helping to prevent HPV infections from occurring. Currently, the FDA has approved the use of two HPV vaccines: a quadrivalent recombinant HPV vaccine (Gardasil) in 2006 and a bivalent HPV vaccine in 2009 (Cervarix).2 The quadrivalent vaccine was approved for girls and women ages 9 to 26 and protects against HPV subtypes 6 and 11—two low-risk HPV subtypes that can cause genital warts—and subtypes 16 and 18—two high-risk HPV subtypes that can cause cervical cancer. This vaccine is given in a three-dose series at months 0, 2, and 6, and should be initiated before women become sexually active for maximum effectiveness.22,35 The quadrivalent vaccine received additional FDA approval for administration in boys and men ages 9 to 26 in 2010.36
The bivalent vaccine is given in a three-dose series as well and is approved for girls and women ages 9 to 25.37 This vaccine offers protection against high-risk HPV subtypes 16 and 18.38
Recent trials have shown that both the quadrivalent and bivalent vaccines are more than 90% effective in preventing the development of precancerous cells from HPV subtypes 16 and 18.2 Although these vaccines provide protection against the two most oncogenic HPV subtypes, routine cytologic testing is still recommended in patients who receive the vaccines because they do not protect against all high-risk subtypes.2,35,38
On the next page: Diagnosis and staging >>