INITIATING ANTIRETROVIRAL THERAPY
It is always helpful to consult an HIV expert prior to initiating antiretroviral therapy, but for patients with no baseline resistance, medical complications, or potential for drug interactions, initial therapy is fairly straightforward. The overall goals of therapy are to maximize the patient’s quality and quantity of life, improve immune function, suppress viral replication, and prevent HIV transmission.15 HIV transmission is significantly reduced when the infected patient is effectively treated and the viral load is suppressed to undetectable. Immune reconstitution as measured by the CD4 count is variable, but most patients will achieve some improvement once viral replication is controlled. Starting antiretroviral therapy is appropriate at any CD4 cell count level, but data regarding the need for therapy as the immune system is depleted are increasingly strong. The strongest data are for patients with CD4 counts below 350 cells/mm3, but large observational studies show benefit to patients starting earlier. There is also a public health benefit of reducing HIV transmission through treatment of patients already infected.25
Therapy cannot, at this time, eradicate the disease, but optimal viral suppression to an undetectable viral load is the most certain indication that therapy is working and should be monitored closely, generally every three or four months. The baseline viral load will respond quickly, and it is helpful to evaluate it shortly after initiating therapy. Scheduling a visit with the patient two weeks after initiating therapy offers the opportunity to perform a viral load test and to discuss any problems the patient has with therapy, including adherence, adverse effects, and any changes in concomitant medications. Alternately, the patient can be scheduled for an HIV RNA test at two weeks, with a clinical visit set for a time when results will be available (generally another week or two). Sharing viral load results is an excellent tool for supporting the patient’s self-efficacy. The dramatic reduction in virus helps both to assure the patient that he or she can successfully control HIV and to reinforce adherence to both medication and clinical follow-up.
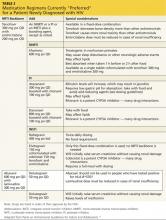
Medication regimens for HIV have become increasingly easy to manage for both the patient and clinician. Most clinicians will generally use those listed as preferred regimens in the HHS guidelines (Table 2). Each recommended regimen contains three antiretroviral medications. Some also contain one additional medicine to pharmacologically boost the activity of one of the other medications by inhibiting metabolism. All recommended regimens include a backbone of two nucleoside reverse transcriptase inhibitors (NRTIs), with either a nonnucleoside reverse transcriptase inhibitor (NNRTI), an integrase strand transfer inhibitor, or a protease inhibitor (PI). All PIs and one of the recommended integrase inhibitor regimens will also include a boosting agent, either ritonavir or cobicistat.15,26
Four nucleoside backbone agents are among those listed as preferred: tenofovir, abacavir, emtricitabine, and lamivudine. Emtricitabine and lamivudine are nearly identical in action and should never be used together. Either may be combined with tenofovir or abacavir, but the availability of fixed-dose coformulations dictates that either tenofovir/emtricitabine (Truvada, also contained in Atripla and Complera, discussed below) or abacavir/lamivudine (Epzicom) is used in practice. Tenofovir is associated with renal toxicity and with osteoporosis. Abacavir is associated with a rare hypersensitivity reaction in patients with a positive HLA-B*5701 mutation. The only abacavir/lamivudine–containing regimen currently included as preferred in the HHS guidelines is with the integrase inhibitor dolutegravir, based on studies done with that combination. With the exception of abacavir, all these medications require an adjustment for patients with renal impairment.15
The one NNRTI-based regimen listed as preferred is a one-pill, once-daily combination of efavirenz, tenofovir, and emtricitabine, coformulated under the trade name Atripla. The efavirenz component of this combination has some teratogenic potential and should generally not be used in women who are likely to become pregnant. Efavirenz has the potential to cause sleep disturbance and other central nervous system (CNS) effects; these often improve after a week or two of therapy. One additional consideration with an efavirenz-containing regimen is the long half-life of the drug relative to other components of the regimen. If a patient is inconsistently adherent, there will be periods when only efavirenz will remain in his or her system, leading to a high potential for the development of resistance. For this reason, it may not be the optimal initial choice for a patient who is known to have difficulty with medication adherence.15
There is another NNRTI-based regimen, a single-pill, once-daily coformulation that includes rilpivirine, tenofovir, and emtricitabine (Complera). It is currently included on the alternative, not the preferred, list of options, primarily because a high rate of virologic failure (failure to achieve an undetectable HIV RNA level) was seen in patients who started the regimen when their HIV RNA levels were greater than 100,000 copies/mL at baseline. In patients with lower HIV RNA levels at baseline, this regimen provides a reasonable alternative for women considering pregnancy or patients with pre-existing psychiatric disorders or sleep disturbances, who are at particular risk for the CNS adverse effects of efavirenz. Like efavirenz, rilpivirine has also been associated with depressive disorders. It must be taken with a full meal (400 cal), so the patient’s eating pattern and access to food should be considered.
Two PI-based regimens are included as preferred: atazanavir and darunavir. Both must be boosted with a low dose (100 mg/d in a treatment-naïve patient) of ritonavir and combined with tenofovir/emtricitabine. While either regimen requires three separate pills daily, all three may be taken together along with food to improve absorption. As a class, boosted PIs provide a strong barrier to resistance and have a similar half-life to the NRTI backbone, making them a good choice for a patient with a history of medication nonadherence. The most common adverse reactions, particularly to the ritonavir portion of the regimen, are gastrointestinal disturbances, such as nausea and diarrhea. PIs are also associated with lipid abnormalities. This is less of a problem with atazanavir than with most other medications in the class. Atazanavir is associated with a benign increase in bilirubin that is generally asymptomatic. A coformulation of darunavir with tenofovir/emtricitabine and boosted with cobicistat rather than ritonavir is likely to be approved and available in the near future, providing the first one-pill, once-daily option for a PI-based regimen.
The newest class of HIV antiretroviral medications are the integrase strand transfer inhibitors: raltegravir, elvitegravir, and dolutegravir. Elvitegravir is available in a fixed-dose, one-pill, once-daily coformulation with tenofovir/emtricitabine and boosted with cobicistat. Raltegravir is currently dosed twice daily in combination with once daily tenofovir/etricitabine, but research on a once-daily formulation is in progress. Raltegravir is metabolized differently from most other medications used for HIV and may be better for use in patients on statins, opioids, oral contraceptives, and many other drugs. Dolutegravir is taken once daily with either tenofovir/emtricitabine or abacavir/lamivudine.15,26
A one-pill, once-daily coformulation of dolutegravir with abacavir/lamivudine will likely be available soon. Though most patients tolerate these drugs well, common adverse effects include nausea, diz-ziness, headache, and insomnia. Dolutegravir and elvitegravir will both cause an initial increase in the serum creatinine level, not associated with renal tubular dysfunction, that will stabilize after a few weeks.26
All preferred regimens are effective and should bring the viral load under control fairly rapidly, although full viral suppression may take weeks or months. Even after viral suppression is achieved, “blips” or temporary increases in the viral load can occur during therapy. However, a persistent viral load exceeding 200 copies/mL may indicate problems with the therapy. Clinicians should assess and reinforce adherence to the regimen, but if the viral load remains detectable, an expert should be
consulted.27
All medications used to control HIV have the potential for drug interactions, both with each other and with other commonly used medications, including OTC and herbal medicines. For this reason, it is important to carefully review all medicines for interactions; a full list may be found at http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adoles cent-arv-guidelines/32/drug-interactions or www.hiv-druginteractions.org/.
On the next page: When to refer and conclusion >>