INTRACEREBRAL HEMORRHAGE: STATINS INCREASE RECURRENCE RISK
In recent years, there has been considerable concern about a statin-induced increased risk for intracerebral hemorrhage (ICH). In a major prospective study in which patients were put on high-dose statin therapy or placebo after an acute ischemic or hemorrhagic stroke, the overall incidence of a recurrent stroke was significantly lower in the statin group.30 Among those who’d had an ICH, however, the recurrence rate was 73% higher for patients taking statins.
A subanalysis that looked only at patients who’d had a hemorrhagic stroke as their initial event (n = 93) found that the absolute risk for recurrent ICH was 15.6% for patients randomized to atorvastatin versus 4.2% for those on placebo.31 Despite being based on a small subset of the original study group, multivariate analysis indicated the increased risk was statistically significant (hazard ratio [HR], 1.69).
A subsequent decision analysis study based on these results proposed that patients with a history of spontaneous deep ICH would need an exceedingly high 10-year cardiovascular event risk (> 40%) for the benefits of statin therapy to outweigh the risk.32 The risk is particularly high for those with a history of lobar ICH, which has an extremely high recurrence rate. However, subsequent retrospective and observational studies have found that patients who were already on statins when the ICH occurred had less severe strokes and more favorable outcomes, with a lower mortality rate at 90 days post-ICH.33-35
A 2010 ICH guideline from the AHA/American Stroke Association states that there is “insufficient data to recommend restrictions on use of statin agents” for patients who have had an ICH.36
The bottom line: Clinicians should carefully evaluate the anticipated cardiovascular risk for patients who have had a hemorrhagic stroke to determine whether statin therapy would be beneficial.
OTHER SERIOUS ADVERSE EFFECTS: WHICH REPORTS ARE ACCURATE?
Statin use has been associated with a number of other serious AEs. Some reports appear to be accurate; others do not hold up after a close look at the evidence.
Malignancy. A potential link between statins and an increased risk for malignancy has been considered for years. A large trial (N = 5,804) from 2002 found a correlation between pravastatin and an increased risk for new cancer diagnoses compared with placebo (HR, 1.25).37 But a 10-year follow-up did not substantiate this finding, and it is now believed that the original result may have been due to chance.38 Numerous other meta-analyses and systematic reviews have found no link between statin use and malignancy.39-41
Cataracts. Potential ocular effects have been widely studied and debated in recent years. Observational studies reporting an association between statin use and cataracts have had conflicting results, with some showing statins as protective42-45 and others finding an increased risk.46,47 However, a recent propensity-score matched analysis found that statin users do indeed have an increased risk for cataracts.48 The authors concluded that for primary prevention, the risk-benefit equation for statin use should include this added risk.48
In addition, a review of the databases of the National Registry of Drug-Induced Ocular Side Effects, the World Health Organization, and the FDA from 1987 to 2008 indicates that statin therapy may also cause diplopia, ptosis, and ophthalmoplegia.49
Peripheral neuropathy. Despite case reports of statin-induced peripheral neuropathy, the NLA’s Neurology Expert Panel states that statins do not appear to cause this condition. If a patient receiving statin therapy develops peripheral neuropathy, a full work-up for other causes should be initiated before modification of statin therapy is considered, the panel advises.28
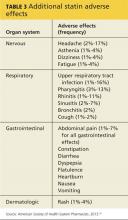
Statins have also been linked to headache and dizziness, respiratory symptoms, gastrointestinal problems, and rash (see Table 3).50
WHICH DRUG? POTENTIAL DIFFERENCES IN STATINS
A meta-analysis with more than 240,000 participants evaluated patients taking seven different statins (atorvastatin, fluvastatin, lovastatin, pravastatin, pitavastatin, rosuvastatin, and simvastatin), looking at AEs of the drugs both collectively and individually.4 As noted earlier, the overall discontinuation rate due to AEs for all statins was 5.7%. Discontinuation rates for each agent were not reported.4
The researchers did report, however, that atorvastatin and rosuvastatin had the highest discontinuation rates; atorvastatin and fluvastatin had the highest incidence of transaminase elevations (OR, 2.6 and 5.2, respectively); and pravastatin and simvastatin appeared to be the best-tolerated and safest statins, with the lowest discontinuation rates. However, higher doses of simvastatin (> 40 mg/d) significantly increased the risk for CK and transaminase elevations (OR, 4.1 and 2.8, respectively),4 as well as the risk for rhabdomyolysis when taken at the highest dose.15,16
Continue for safety concerns >>