HbA1c target: How low should you go?
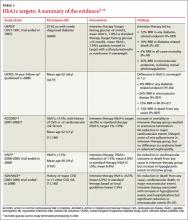
The Diabetes Control and Complications Trial (DCCT), published nearly 20 years ago, studied patients with type 1 diabetes, and found that intensive insulin therapy (HbA1c ≤6%) delayed the onset of retinopathy, nephropathy, and neuropathy.2 However, there was an important adverse effect of such intensive therapy: Patients in this group suffered from severe hypoglycemic episodes 3 times more frequently than those in the usual care group. Nonetheless, the microvascular benefits of intensive control observed in those with type 1 diabetes were thought to be similar for patients with type 2 diabetes.
The United Kingdom Prospective Diabetes Study (UKPDS), published in 1999, was the first major study to investigate targets for glucose control in patients with type 2 diabetes.3 Participants treated intensively (mean HbA1c goal, 7%) had a 25% reduction in microvascular complications, including the need for retinal photocoagulation, com- pared with those on standard control (mean HbA1c, 7.9%). There was also a nonsignificant trend toward a reduction in macrovascular complications in the intensive therapy group, but no difference in overall mortality rate.3
A 10-year follow-up of the UKPDS showed that while baseline differences in HbA1c between the 2 groups were lost by one year, reductions in microvascular complications continued to occur in the intensive treatment group.4 Reductions in myocardial infarction (MI) and death emerged over time, a possible legacy effect (ie, the result of intense treatment early in the course of the disease).
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, published in 2008, studied patients at risk for CVD, defined by either a prior history of CVD or ≥2 other cardiovascular risk factors.5 Participants, all of whom had poorly controlled type 2 diabetes (mean HbA1c, 8.1%), were randomized to either intensive treatment (HbA1c goal, <6%) or standard therapy (HbA1c goal, 7%-7.9%). The study was discontinued after a mean follow-up of 3.5 years, when those in the intensive therapy group were found to have a higher mortality rate.5
The rate of nonfatal MI reported by the ACCORD trial was lower in the intensive therapy group, however, and participants in this group also had delayed onset of microalbuminuria.6 No differences were seen in serum creatinine concentrations, advanced nephropathy, diabetic eye complications, or nonfatal stroke. Five-year follow up confirmed an increased mortality rate in the intensive therapy group,7 the result of severe hypoglycemia.8
The Veterans Affairs Diabetes Trial (VADT) randomized patients with poorly controlled type 2 diabetes to intensive or standard therapy.9 At 6 months, the intensive therapy group’s HbA1c averaged 6.9%, compared with 8.4% for the standard therapy group. Except for a delay in the progression of albuminuria, no significant effects of intensive therapy were found: Rates of other microvascular complications, major cardiovascular events, and death were similar.9 It should be noted that the VADT involved fewer participants and shorter follow-up than the other trials cited (TABLE 1),3-10 which may have affected its findings.
The Action in Diabetes and Vascular Disease (ADVANCE) trial, which included participants with either a history of major CVD or ≥1 other CVD risk factors, compared an intensive control group (mean HbA1c, 6.5%) with a standard care group (mean HbA1c, 7.3%)—with mixed results.10 Microalbuminuria occurred less frequently in the intensive therapy group, but hypoglycemia and hospitalization increased. No reduction in death from any cause, in cardiovascular death, or in major macrovascular events was found.
Continue for what experts recommend >>