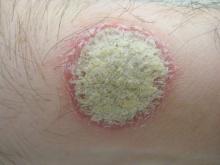
Guselkumab, a monoclonal antibody that specifically inhibits interleukin-23 signaling, was safe and more effective than placebo or adalimumab against plaque psoriasis in a phase II clinical trial, according to a report published online July 9 in the New England Journal of Medicine.
The 1-year randomized double-blind study, funded and administered by the maker of guselkumab, involved 293 adult patients treated at 31 sites in North America and 12 in Europe. The median duration of disease was 19 years. Study participants were randomly assigned to receive one of five subcutaneous doses of the agent (5 mg, 15 mg, 50 mg, 100 mg, or 200 mg) at two different dosing intervals (208 patients), matching placebo (42 patients), or adalimumab (43 patients) for 40 weeks and were followed for an additional 12 weeks. At 16 weeks, patients in the placebo group crossed over to receive 100 mg of guselkumab every 8 weeks, said Dr. Kenneth B. Gordon of Northwestern University, Chicago, and his associates.
The primary efficacy endpoint – the proportion of patients at week 16 with a Physician’s Global Assessment score of 0 or 1 – was significantly higher in every guselkumab dosing group (34%, 61%, 79%, 86%, and 83%, respectively) than in the placebo group (7%). In addition, the proportion of patients showing a 75%, 90%, or 100% improvement in Psoriasis Area and Severity Index score was significantly higher in each guselkumab group than in the placebo group. Scores on the Dermatology Life Quality Index also showed significantly greater improvement from baseline to week 16 in the guselkumab groups.
The findings were similar when patients taking the higher doses of guselkumab were compared with those taking adalimumab. At week 16, the proportion of patients with a PGA score of 0 or 1 was 58% with adalimumab, compared with 79%, 86%, and 83% at the highest doses of guselkumab (50 mg, 100 mg, or 200 mg). And at week 40, the proportion of patients with a PGA score of 0 or 1 was significantly higher in those taking 50 mg (71%), 100 mg (77%), or 200 mg (81%) guselkumab than in patients taking adalimumab (49%). The response to guselkumab was noted at the earliest patient assessment at week 4, peaked at week 20, and was maintained through week 40, Dr. Gordon and his associates said (N. Engl. J. Med. 2015 July 9 [doi:10.1056/NEJMoa1501646]).
The rates of adverse events, serious adverse events, and infections were similar across all the treatment groups. At week 16, infections were reported in 20% of patients taking guselkumab, 14% of those taking placebo, and 12% of those taking adalimumab. At final follow-up, infections were reported in 30% of patients taking guselkumab and 35% taking adalimumab. This study had a limited ability to assess uncommon adverse events; three patients taking guselkumab had major cardiovascular events during treatment, but “it is difficult to interpret the significance” of such events, the investigators said.
They added that larger and longer-term phase III trials are now underway to further assess the efficacy and safety of guselkumab for this indication. Moreover, their study results “validate the use of interleukin-23 as a therapeutic target by showing that therapy involving selective antagonism of this single cytokine in patients with moderate to severe plaque psoriasis is highly efficacious, as compared with adalimumab therapy,” Dr. Gordon and his associates said.