MONTEREY, Calif. — Using imiquimod on nodular basal cell carcinomas on the nose before Mohs surgery failed to simplify the surgery or reduce costs and significantly increased local adverse reactions, in a randomized, controlled study of 28 patients.
"Doctors across the country are using imiquimod 'off label' to treat BCCs on the nose, and they should know these findings before doing that," said Dr. David F. Butler.
The study was inspired by a patient who refused surgery or radiation for her nodular, nasal BCC and was treated with good results using imiquimod, he said at the annual meeting of the American Society for Mohs Surgery. Perhaps, he reasoned, adjunctive imiquimod might reduce similar tumors before treatment with Mohs surgery.
"I was surprised that imiquimod did not reduce the number of stages, reduce the cost of Mohs surgery, or reduce the cost of repair," said Dr. Butler, chair of dermatology at the Scott and White Clinic and professor of internal medicine at Texas A&M University, both in Temple, Tex.
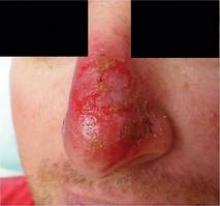
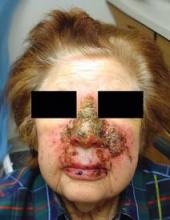
Of the 31 patients who enrolled in the study, 3 dropped out, 2 because of local adverse events and 1 because of other illness. Among the 28 patients who completed the study, 10 of 12 (83%) in the imiquimod arm and 4 of 16 (25%) in the control arm developed local adverse events after 3 weeks of treatment, including redness, blisters, erosions, and crusting. After 6 weeks, the same number in the imiquimod group and two patients in the control group (12%) had local adverse reactions. The differences between the groups were statistically significant.
The frequency of local adverse events limits the usefulness of imiquimod as adjunctive therapy in these cases, he said.
Only one stage of Mohs surgery was needed for 11 patients in each group. Two stages were needed for one patient in the imiquimod arm (8%) and five patients in the control arm (31%). Surgical defect sizes averaged 88 mm2 in the imiquimod arm and 100 mm2 in the control arm (Dermatol. Surg. 2009;35:24-9).
A larger study might show a difference, he acknowledged. The current study did not include the cost of imiquimod, so even if a larger study finds that adjunctive imiquimod therapy reduces the number of Mohs stages needed, the medication cost might negate any cost savings in the surgery.
Patients applied 5% imiquimod cream or vehicle to the tumor five nights a week for 6 weeks and covered it with a bandage supplied by investigators. One month after stopping the imiquimod, they underwent Mohs surgery.
Dr. Butler said in an interview that he was surprised by the "inordinately high" proportion of patients with tumor remaining at the time of Mohs surgery after imiquimod treatment.
Only 5 of 12 patients (42%) in the treatment group had complete clearance of the tumor after imiquimod therapy, with tumor absent (presumably destroyed) in the first stage block. "That's a relatively low number when you compare it to the 80% cure rate that you get when using imiquimod to treat superficial BCCs on the trunk or extremities," he said. "My concern is that nodular BCCs on the nose may be a different problem."
Imiquimod was approved in 2004 to treat superficial BCC on the trunk and extremities, with histologic cure rates of 79%-82% at those sites, previous studies have shown. Clearance rates have been lower, however, for nodular BCCs, with reports ranging from 65% to 76%, he noted. In addition, the most common site for BCCs is not the trunk or extremities but the nose, accounting for 25%-30%.
Dr. Butler advised against using imiquimod as a stand-alone therapy for nodular BCCs on the nose, but said it may be a reasonable option for patients who cannot or will not undergo other treatments.
"We do recommend, however, that if you're going to use imiquimod as a single therapy for nodular BCCs on the nose, that once you finish the treatment, that you go back and do a little biopsy of the area to document that the cancer is gone," he added.
Graceway Pharmaceuticals and 3M funded the study. Dr. Butler reported that he had no conflicts of interest.