To the Editor:
Anti–tumor necrosis factor (TNF) α treatments have radically improved the management of chronic inflammatory conditions, including rheumatoid arthritis, ankylosing spondylitis, psoriasis and psoriatic arthritis, and bowel diseases (eg, Crohn disease, ulcerative colitis [UC]). Because the number of patients treated with these agents has increased, uncommon adverse reactions have increasingly occurred. Cutaneous adverse reactions that have been reported with anti-TNF agents include immediate injection-site reaction, systemic infusion reactions, and delayed reactions.1 Among the delayed adverse reactions, psoriatic and eczematous eruptions as well as cutaneous infections are the most common, while cutaneous adverse effects related to an immune imbalance syndrome including vasculitis; lupuslike, lichenlike, and granulomatous eruptions; and skin cancer rarely are observed.1 Although most of the cutaneous adverse effects do not require anti-TNF treatment discontinuation and are resolved with symptomatic treatment, anti-TNF therapy must be stopped in more severe cases. We report the case of severe Henoch-Schönlein purpura (HSP) following treatment with infliximab.
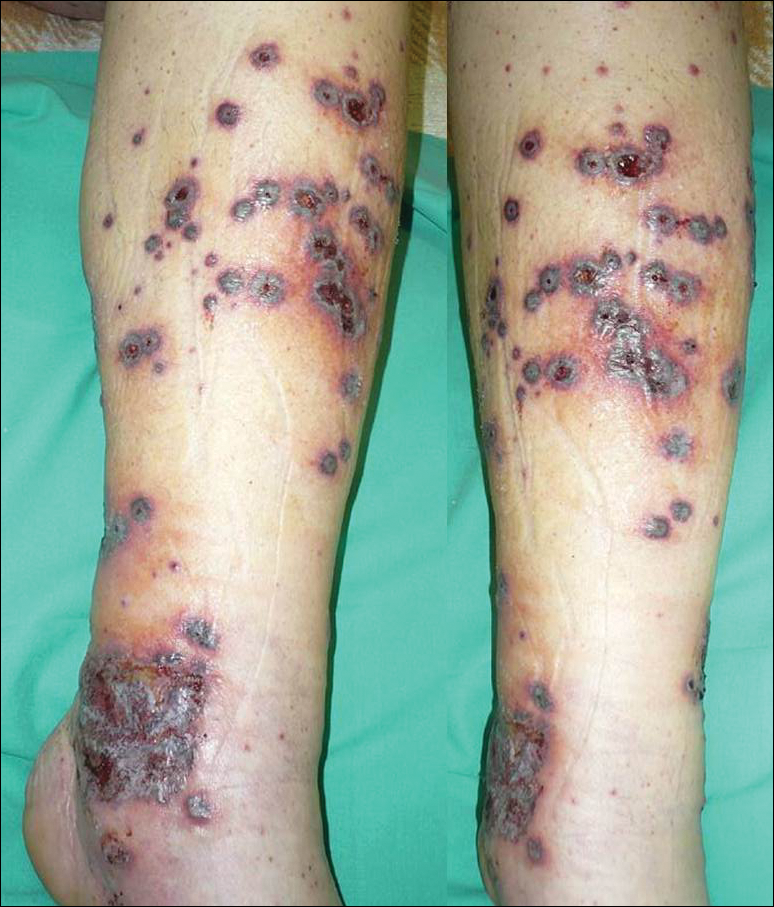
A 46-year-old man who was a nonsmoker with quiescent UC on infliximab for 30 months presented with palpable necrotic purpura on both legs (Figure) and arms as well as the abdomen of 10 days’ duration, along with diffuse joint pain and swelling. He had no history of infectious or gastrointestinal symptoms. The last infliximab infusion was performed 6 weeks prior to developing the purpura. His UC was diagnosed 10 years prior to the current presentation and was not associated with any extragastrointestinal manifestations. Since diagnosis, UC had failed to respond to therapies such as azathioprine, cyclosporine, and purinethol. The complete blood cell count was normal. The C-reactive protein level was 18.7 mg/L (reference range, <5 mg/L) and the erythrocyte sedimentation rate was 30 mm/h (reference range, 0–20 mm/h). Electrolytes, urea, creatinine clearance, and liver function were normal, and a chest radiograph and radiographs of the swollen joints were unremarkable. The total IgA level was elevated at 4 g/L (reference range, 0.7–4 g/L), with IgG and IgM levels within reference range. There was no hematuria or proteinuria on urinalysis. Tests for antinuclear antibodies, rheumatoid factor, circulating immune complexes, and antineutrophil cytoplasmic antibody were negative. Total complement, C3, and C4 levels also were normal. A skin biopsy confirmed a leukocytoclastic vasculitis of small vessels with C3 deposition. Serologic tests for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus were negative. Based on these findings, the diagnosis of HSP was made. Systemic corticosteroids—120 mg daily of intravenous methylprednisolone for 3 days, followed by 1 mg/kg daily of oral prednisone for 2 weeks—were then introduced with rapid clinical improvement. Henoch-Schönlein purpura and joint symptoms completely resolved, but UC relapsed with bloody diarrhea and severe abdominal pain. Oral prednisone was maintained (1 mg/kg daily). Because of the severity of cutaneous vasculitis (HSP), a multidisciplinary decision was taken to definitively stop the anti-TNF agents and to first add azathioprine (2 mg/kg daily for 2 months), then subcutaneous methotrexate (25 mg weekly). Colonoscopy did not show any dysplasia or adenocarcinoma and confirmed the diagnosis of UC. After 6 months of combined therapy, UC was still active and we decided to perform a total colectomy with ileostomy formation. Complete remission of UC was obtained and maintained after 28 months of follow-up.