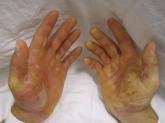
The U.S. Food and Drug Administration has granted breakthrough therapy status to Abeona Therapeutics’s EB-101 gene therapy program for patients with recessive dystrophic epidermolysis bullosa, according to a company statement.
The breakthrough therapy designation will expedite the phase 3 trial, intended to start in 2018, and the approval process and make this therapy available to patients who have few or no other treatment options for this serious and painful condition.
In the phase 1/2 clinical trial, EB-101, an autologous, ex vivo gene-corrected cell therapy in which the COL7A1 gene is inserted into a patient’s own skin cells, was administered to nonhealing chronic wounds. When compared with untreated control wounds, treated wounds were significantly healed (greater than 50% healed) for more than 2 years after administration in all 128 evaluated patients.The experimental treatment “utilizes a patient’s own cells and genetically engineer[s] them to produce the correct version of collagen, which helps hold skin on to the body, thereby reducing the number of painful blisters caused by injury and improving wound healing,” Timothy J. Miller, PhD, CEO and president of Abeona, said in a statement. “We are grateful that the FDA has recognized the promising clinical data from the EB-101 program with Breakthrough Therapy designation.”