Results
Study Protocol Amendments
Given the number of diabetic patients in the US veteran population, we anticipated that there would be enough participants meeting the inclusion and exclusion criteria; however, because of the difficulty with recruitment, the initial study criteria were modified. The study was initially designed to incorporate DFUs with a minimum size of 1.0 cm2.12The study investigators noted that within the veteran population, many diabetic patients with DFUs had ulcers that were too small to meet the inclusion criteria; thus, these patients could not be captured in the trial. However, those small ulcers would stall for months, which prompted the decision to change this major exclusion criterion to allow patients with a wound size greater than 0.5 cm2 (versus 1.0 cm2) to be recruited. Enrollment of participants also was extended to include nonveterans.
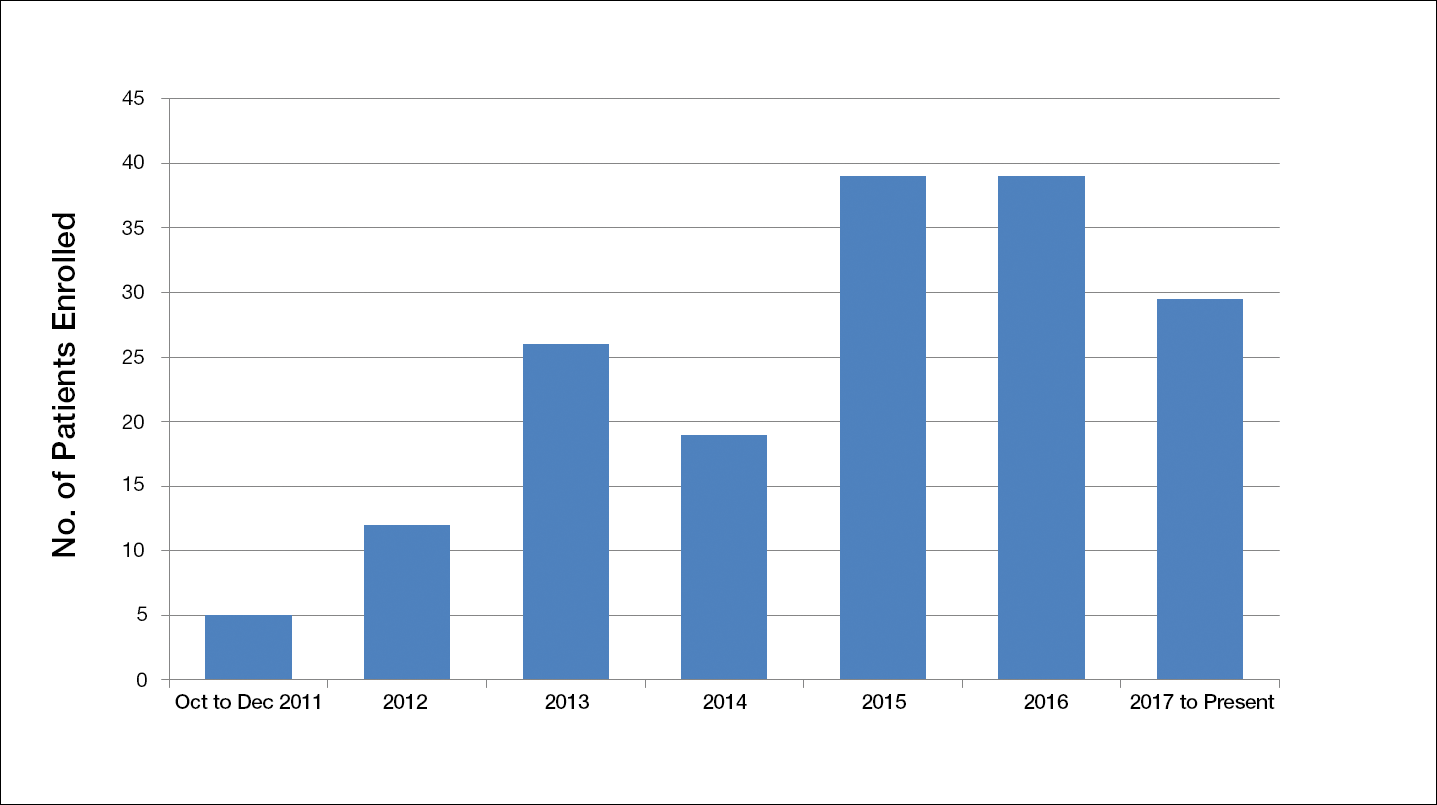
Another limiting criterion was the percentage of total hemoglobin level for hemoglobin A1C (HbA1C). The study was originally established to include participants with an HbA1C level of 10% of total hemoglobin or below.12 Unfortunately, the majority of the potential participants had values substantially higher, and thus could not be enrolled in the trial, requiring another amendment to the study protocol in 2014, which was approved to include patients with an HbA1C level less than 12% of total hemoglobin. This change contributed considerably to the noted increase in enrollment rates in 2015, which almost doubled relative to enrollment under the original exclusion criteria (Figure).
The study has screened more than 600 patients. Among them, 137 were assessed for eligibility; 71 were excluded for various reasons, including screen failure (eg, decrease in wound size by >40% during the 2-week screening phase), loss to follow-up, and adverse events. Sixty-six participants reached the primary outcome at week 12, while 55 participants completed the study (19 in the SOC group; 18 in the cellular matrix group; 18 in the acellular matrix group).
We have stopped enrolling patients from all sites and the community, as we have reached our target enrollment.
Comment
One of the challenges of clinical trials is the recruitment of an adequate number of participants within an appropriate time frame, which is explained by Lasagna’s Law,18 a well-described phenomenon whereby the investigator overestimates the number of potential participants available to meet the inclusion criteria. This so-called funnel-effect was partly encountered in our selection process. A review of the veteran population with DFUs seemed to be more than adequate to fulfill the sample size; however, some important participant-related factors also played a substantial role. The criterion for minimum ulcer size of 1.0 cm2 was comparable to other trials8 but was a major limiting factor in our study. Many participants already were established with either the podiatry or multispecialty wound clinics, and they had small DFUs, which were stalling for months. Thus, by decreasing the lesion size needed for inclusion, our trial benefited from this subset population.
In addition, the Veterans’ Affairs network centralizes health information, making it readily available to all providers participating in their care. As a result, patients with diabetes mellitus typically are seen by a primary care physician along with an endocrinologist, a diabetic nurse, and/or a dietician. Despite the collaboration with an interdisciplinary team, the glycemic control of the participants remains an issue along with other psychosocial factors that are deterrents in patient compliance. As a result, patients with poorly controlled diabetes and an HbA1C level above 10% (and less than 12%) of total hemoglobin who were initially excluded from the study were reincluded after modifying the inclusion criteria. Some patients were interested in joining the study, but physical limitations (eg, impaired mobility) prompted their decision not to join the trial, even though they met all the inclusion criteria.
As far as research-related factors that could affect participation, it is notable that most of the patients were retired; thus, the interventions did not cause additional burden of taking time off from work or loss of productivity. Although randomization could be a deterrent in many clinical trials, the majority of patients were willing to participate without demanding to be assigned to a particular treatment group. Some research-related factors that were an impediment to patient enrollment included the time to travel and the associated expenses, but our trial was designed to offer a small stipend for travel reimbursement (up to $400) to mitigate such factors.
There are many factors that are intertwined and can lead to enrollment and/or attrition rates. It was critical for our team to make some adjustment without compromising the controlled nature of a randomized trial.
Acknowledgment
The authors wish to acknowledge Huong Le, DPM, MPH, who was the coauthor of the study protocol.