Biologic TNF-α Antagonists
Following the PTX case report and the thalidomide trial, there was increased interest in using newer-generation TNF-α inhibitors, such as the monoclonal antibody infliximab or the fusion protein etanercept, in the treatment of TEN. To date, there are 10 known published case reports,11,12,15-21,23 3 case series,13,14,22 and 1 trial24 describing the use of these agents; however, treatment protocols vary. Categories of treatment protocols include the use of TNF-α inhibitors as monotherapy, following failure of other systemic agents, and in combination with other systemic therapies.
TNF-α Inhibitors as Monotherapy
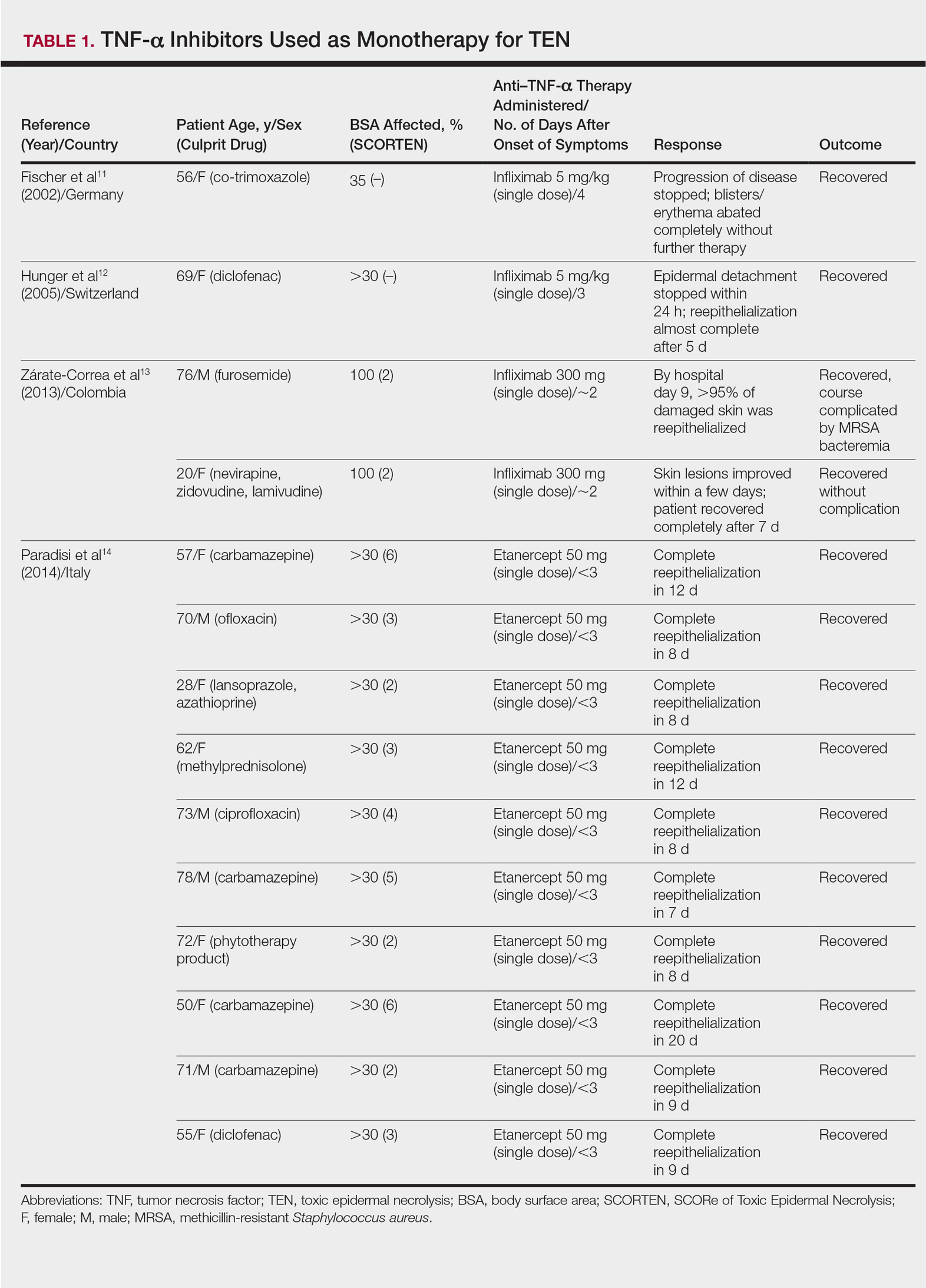
Review of the literature yielded 2 case reports using infliximab monotherapy11,12 and 2 case series using infliximab or etanercept monotherapy13,14 with a total of 14 patients (Table 1). Fischer et al11 was the first of these reports to describe a patient successfully treated with supportive care and a single dose of infliximab 5 mg/kg. The dose was given 4 days after the onset of symptoms, and the rapid progression of the disease was stopped, with complete recovery in less than 4 weeks.11 Hunger et al12 also described the successful treatment of a patient using a similar protocol: a single dose of infliximab 5 mg/kg given 3 days after symptom onset. Epidermal detachment was abated within 24 hours and the patient had almost complete reepithelialization within 5 days.12 In a case series published by Zárate-Correa et al,13 2 patients with near 100% body surface area involvement were successfully treated with a single dose of infliximab 300 mg. Although both of these patients experienced fairly rapid recoveries, one patient’s course was complicated by methicillin-resistant Staphylococcus aureus bacteremia.13 Paradisi et al14 described 10 consecutive patients treated with a single dose of etanercept 50 mg given within 6 hours of hospital admission and within 72 hours of symptom onset. The SCORTEN (SCORe of Toxic Epidermal Necrolysis) scale—a severity-of-illness assessment for TEN based on body surface area involvement, comorbidities, and metabolic abnormalities—was used to predict mortality in these patients. The investigators reported an expected mortality of 46.9%; however, the observed mortality was 0%, and there were no reported infections.14
TNF-α Inhibitors Following Failure of Other Systemic Agents in TEN
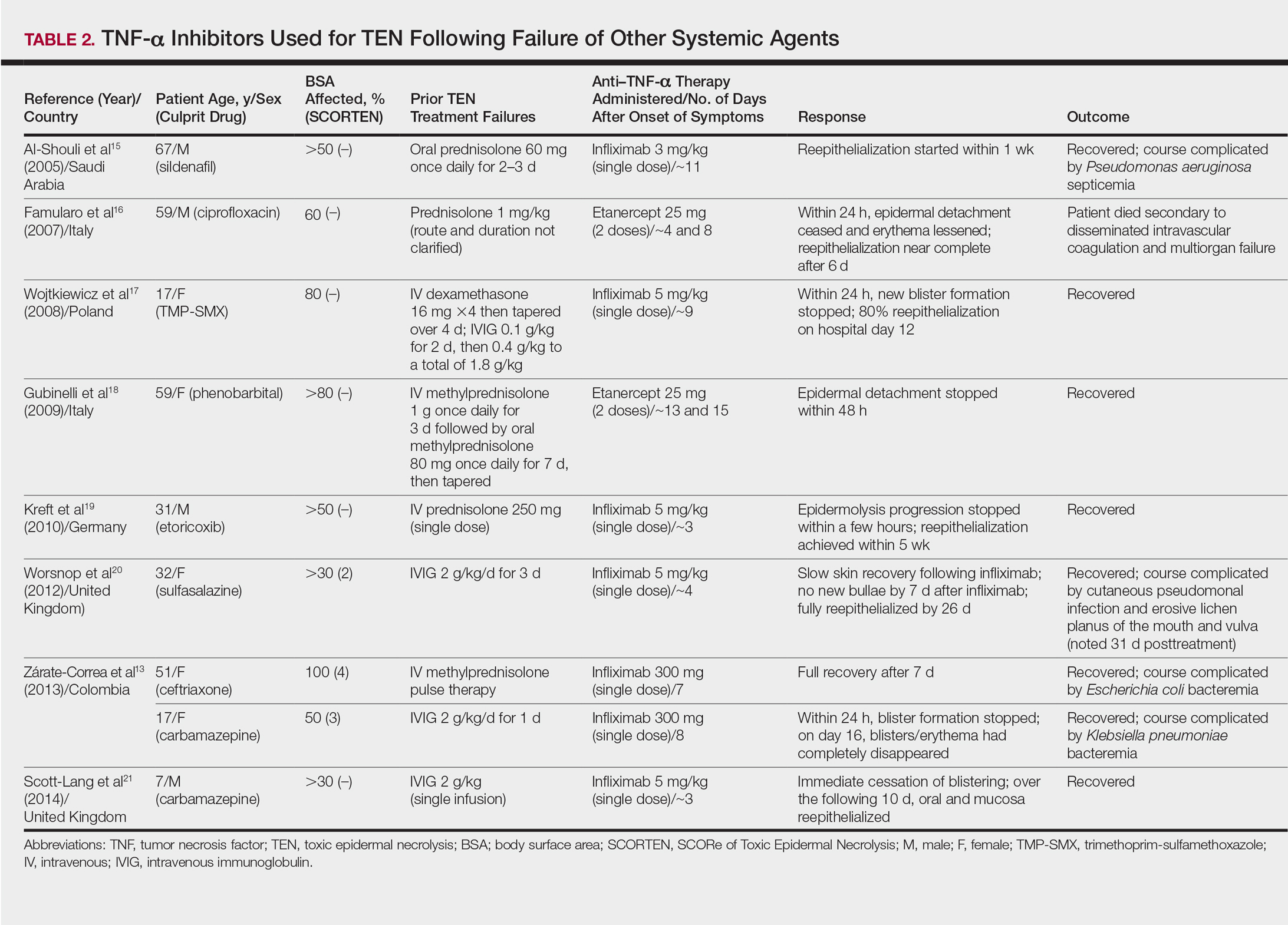
Seven case reports and 1 case series using anti–TNF-α therapy following failure of other systemic agents were reviewed for a total of 9 patients (3 pediatric/adolescent patients, 6 adult patients)(Table 2).13,15-21 Seven patients were treated with infliximab,13,15,17,19-21 and the remaining 2 patients were treated with etanercept.16,18 All patients were treated initially with corticosteroids and/or IVIG. In each case, anti–TNF-α therapy was introduced when prior treatment failed to halt the progression of TEN. Most reports claimed a rapid and beneficial response to anti–TNF-α therapy. Eight of 9 (88.9%) patients recovered.13,15,17-21 Famularo et al16 described 1 patient who was treated with 2 doses of etanercept following prednisolone but died on the tenth day of hospitalization secondary to disseminated intravascular coagulation and multiorgan failure; however, the patient reportedly had near-complete reepithelialization of the skin on the sixth day of the hospital course.16 Of the 8 surviving patients, 3 (37.5%) experienced hospital courses complicated by nosocomial gram-negative bacteremia, including Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae.13,15 Interestingly, a patient described by Worsnop et al20 developed erosive lichen planus of the mouth and vulva 31 days after infliximab infusion.
Combination of TNF-α Inhibitor With Other Systemic Agents in TEN
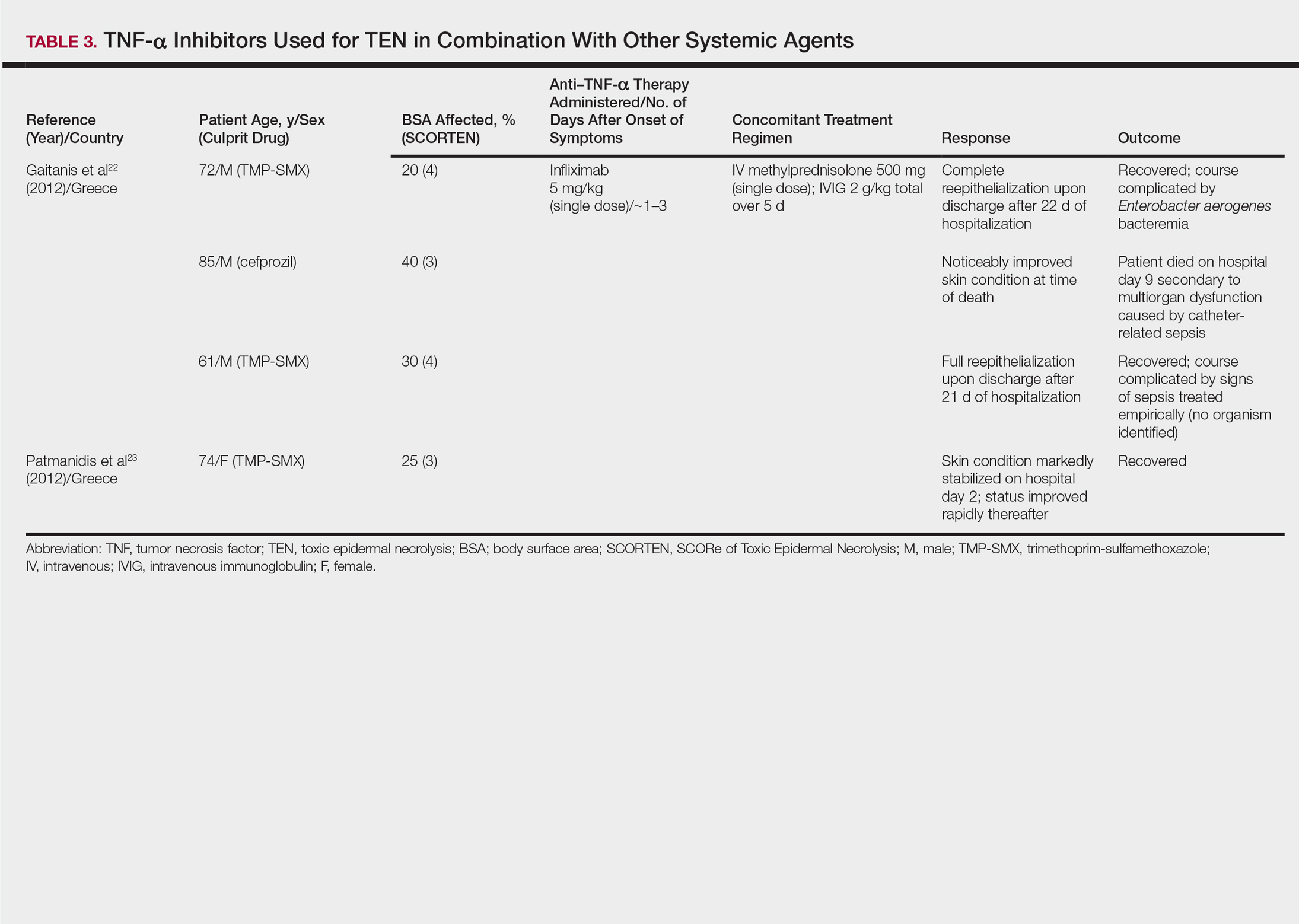
One case series22 and 1 case report23 using infliximab in combination with other systemic therapies were reviewed with a total of 4 patients (Table 3). Both reports utilized the same treatment protocol, which consisted of a single bolus of intravenous methylprednisolone 500 mg followed by a single dose of infliximab 5 mg/kg and then IVIG 2 g/kg over 5 days. Three of 4 (75%) patients recovered.22,23 Gaitanis et al22 reported a patient who died on the ninth day of hospitalization secondary to multiorgan dysfunction caused by a catheter-related bacteremia. Similar to the patient described by Famularo et al,16 this patient also was noted to have remarkably improved skin prior to death. Two of the other 3 patients that survived had their hospital course complicated by infection, requiring antibiotics.22 In the Gaitanis et al22 series, the average predicted mortality according to a SCORTEN assessment was 50.8%; however, mortality was observed in 33.3% (1/3) of patients in the case series.
N-Acetylcysteine and Infliximab
The combination of NAC and infliximab was studied in a randomized controlled trial using TNF-α inhibition in TEN.24 In this study, 10 patients were admitted to a burn unit and treated with either 3 doses of intravenous NAC (150 mg/kg per dose) plus 1 dose of infliximab 5 mg/kg or NAC alone. Unlike some of the previously described articles, Paquet et al24 utilized an illness auxiliary score (IAS), which predicts both disease duration and mortality. An IAS was taken at admission and again 48 hours after completion of NAC and/or infliximab administration. The mean clinical IAS score was reported to have remained unchanged at treatment completion in the NAC group and slightly worsened in the NAC-infliximab group. One patient died in the NAC group and 2 patients died in the NAC-infliximab group, each due to infection. These fatalities corresponded to a mean mortality of 20% in the NAC-treated group and 40% for the NAC-infliximab group. To compare, the predicted mortalities based on the IAS were 20.4% and 21.4%, respectively.24