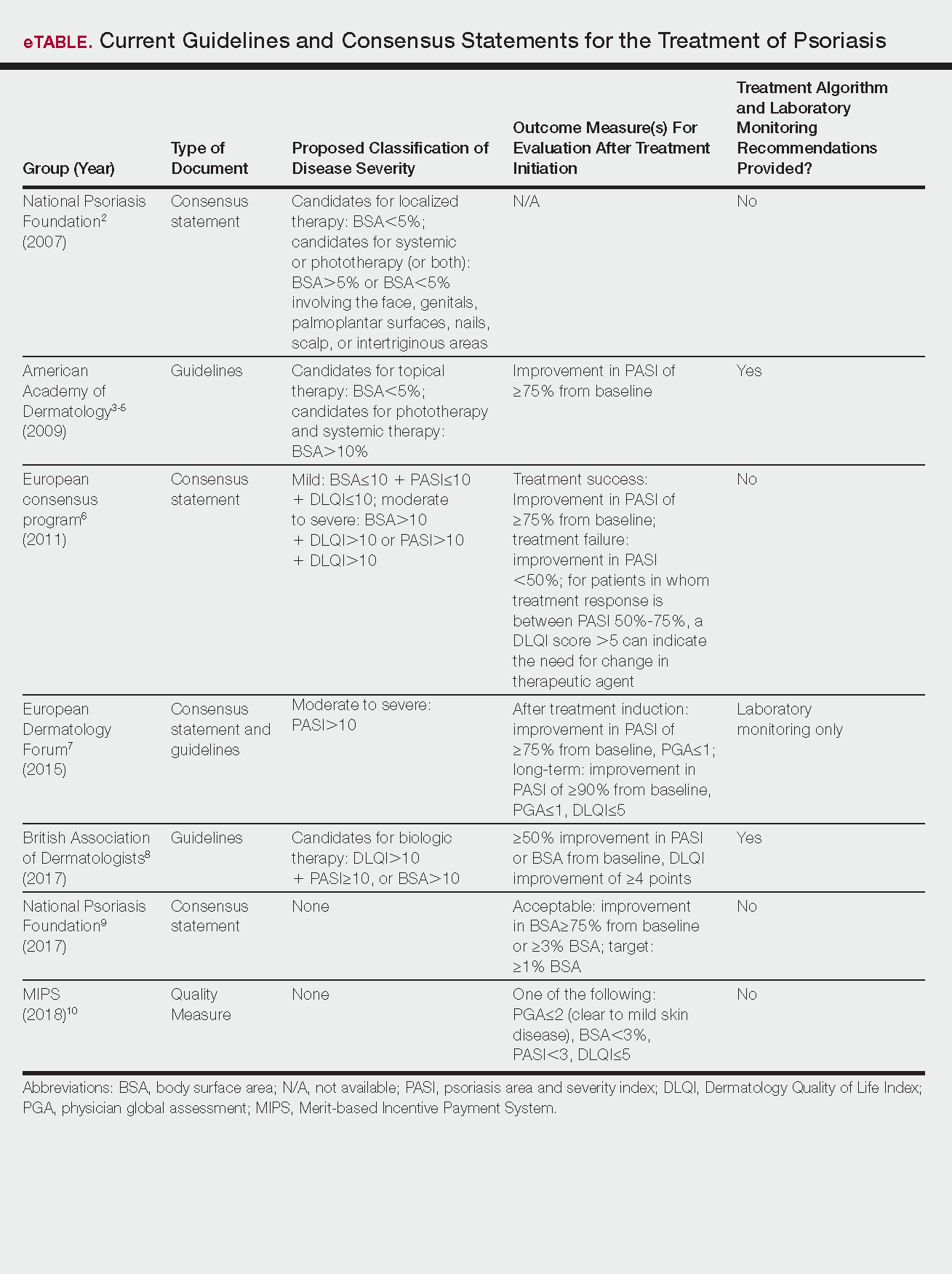
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10
Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15