To the Editor:
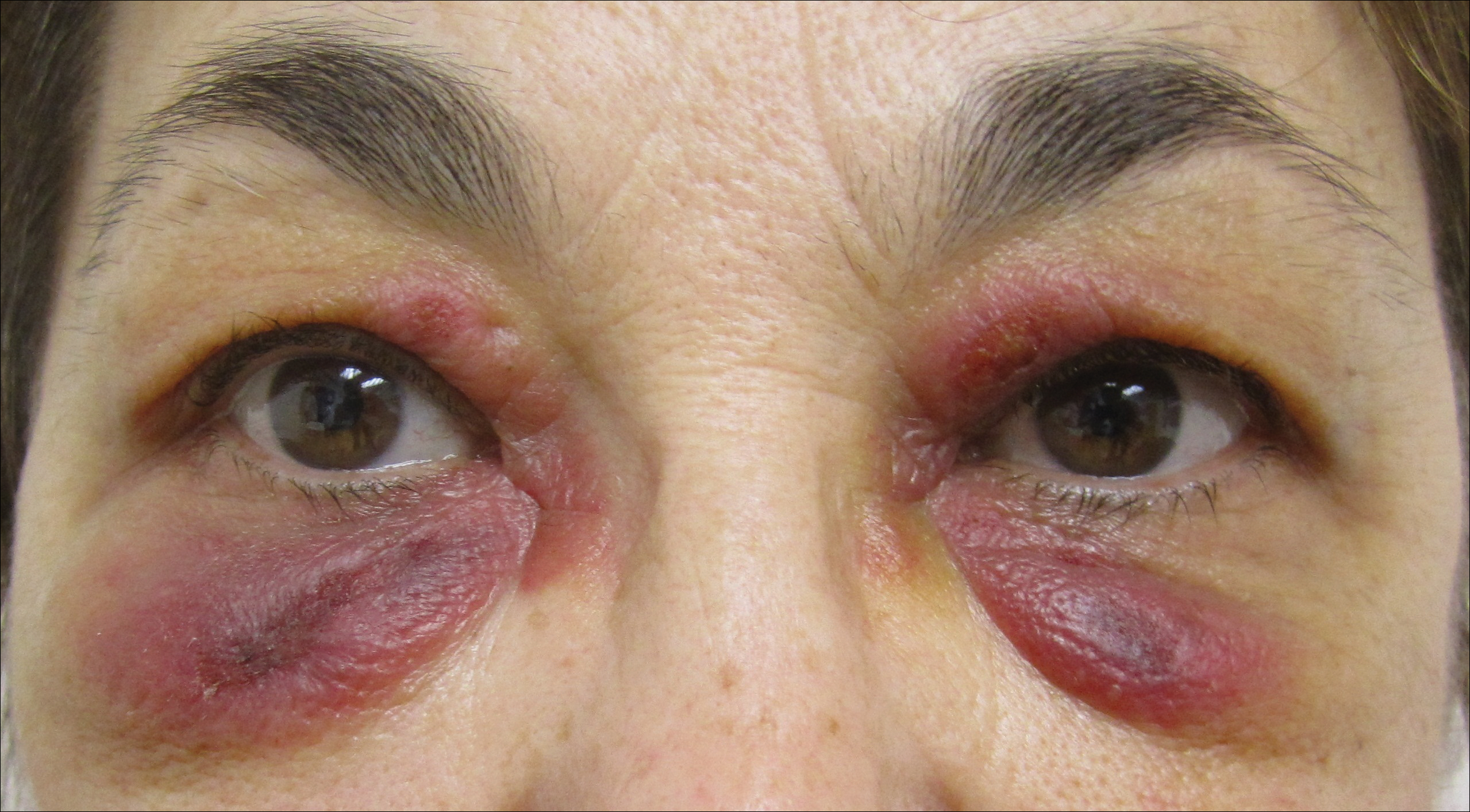
A 65-year-old woman with B-cell marginal zone lymphoma presented with asymptomatic swelling and redness of the upper and lower eyelids of 1 week’s duration that was unresponsive to topical corticosteroids for presumptive allergic contact dermatitis. She denied any lip or tongue swelling, abdominal pain, or difficulty breathing or swallowing. Diagnosis of acquired angioedema (AAE) was confirmed on laboratory analysis, which showed C1q levels less than 3.6 mg/dL (reference range, 5.0–8.6 mg/dL), complement component 4 levels less than 8 mg/dL (reference range, 14–44 mg/dL), and C1 esterase inhibitor (C1-INH) levels of 3 mg/dL (reference range, 12–30 mg/dL).
A review of the patient’s medical record showed chronic thrombocytopenia secondary to previous chemotherapy. It was determined that the patient’s ecchymosis and purpura of the eyelids was secondary to a low platelet count resulting in bleeding into the area of angioedema (Figure). Serum protein electrophoresis did not demonstrate a monoclonal spike, and flow cytometry showed persistent B-cell leukemia without evidence of an aberrant T-cell antigenic profile. The edema and purpura of the eyelids spontaneously resolved over days, and the patient has had no recurrences to date. She was prescribed icatibant for treatment of future acute AAE attacks.
The common pathway of AAE involves the inability of C1-INH to stop activation of the complement, fibrinolytic, and contact systems. Failure to control the contact system leads to increased bradykinin production resulting in vasodilation and edema. Diagnosis of hereditary angioedema (HAE) types 1 and 2 can be confirmed in the setting of low complement component 4 and C1-INH functional levels and normal C1q levels; in AAE, C1q levels also are low.1,2
The malignancies most frequently associated with AAE are non-Hodgkin lymphomas (eg, nodal marginal zone lymphoma, splenic marginal zone lymphoma), such as in our patient, as well as monoclonal gammopathies.2 Triggers of AAE include trauma (eg, surgery, strenuous exercise), infection, and use of certain medications such as angiotensin-converting enzyme inhibitors and estrogen, but most episodes are spontaneous. Swelling of any cutaneous surface can occur in the setting of AAE. Mucosal involvement appears to be limited to the upper airway and gastrointestinal tract. Edema of the upper airway mucosa can lead to asphyxiation. In these cases, asphyxia can occur rapidly, and therefore all patients with upper airway involvement should present to the emergency room or call 911. Pain from swelling in the gastrointestinal tract can mimic an acute abdomen.3
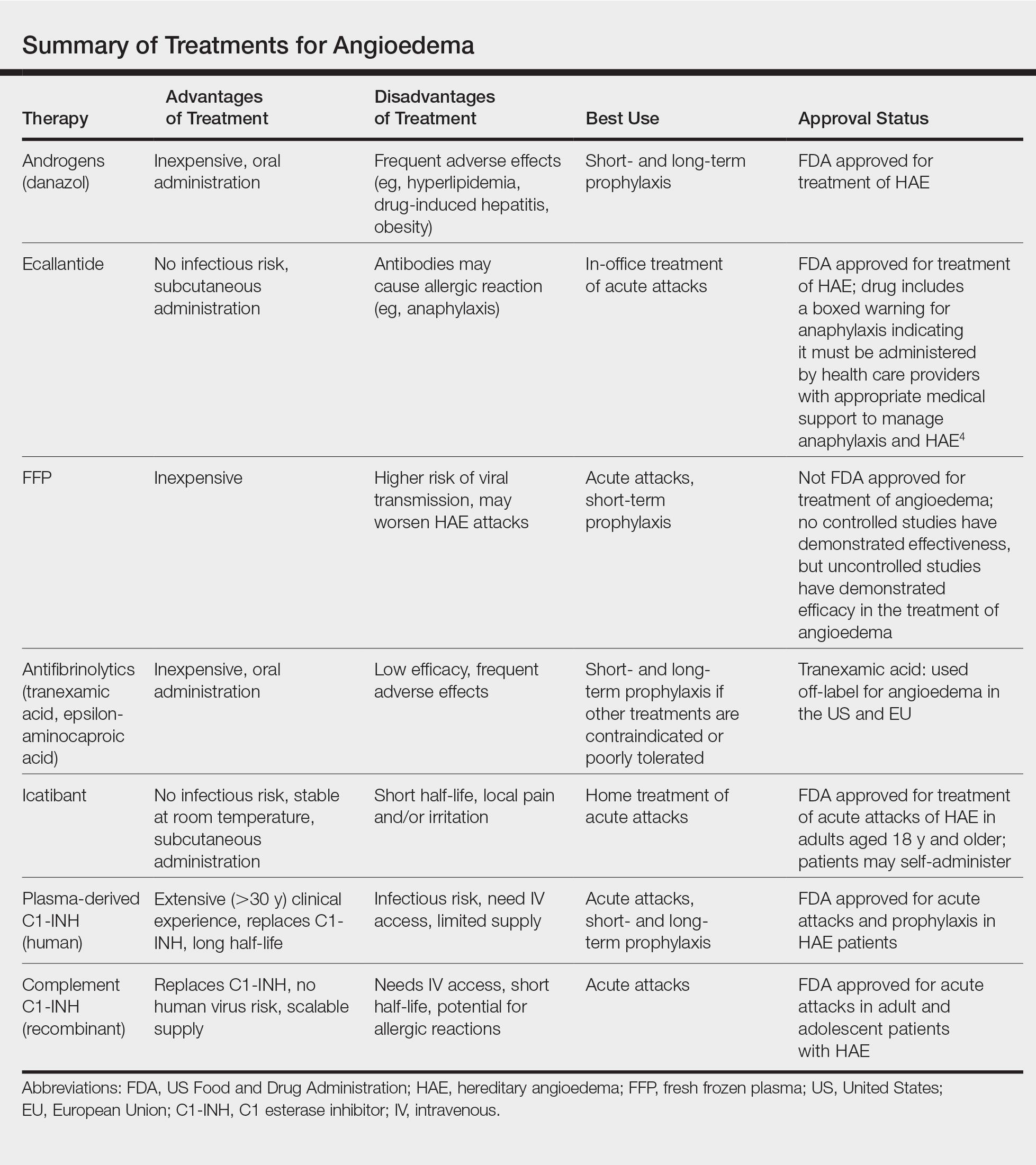
Newly developed targeted therapies for HAE also appear to be effective in treating AAE. A summary of available treatments for angioedema is provided in the Table. Human plasma C1-INH can be used intravenously to treat acute attacks or can be given prophylactically to prevent attacks, but large doses may be necessary due to consumption of the protein.1,3 The risk of bloodborne disease as a result of treatment exists, but screening and processing during production of the plasma makes this unlikely. Ecallantide is a reversible inhibitor of plasma kallikrein.1,3 Rapid onset and subcutaneous dosing make it useful for treatment of acute AAE attacks. Because anaphylaxis has been reported in up to 3% of patients, ecallantide includes a boxed warning indicating that it must be administered by a health care professional with appropriate medical support to manage anaphylaxis and HAE.4 Icatibant is a selective competitive antagonist of bradykinin receptor B2. It can be administered subcutaneously by the patient, making it ideal for rapid treatment of angioedema.1,3 Adverse events include pain and irritation at the injection site.
The most appropriate therapy for AAE is treatment of the underlying malignancy. Recognition and proper treatment of AAE is essential, as bradykinin-induced angioedema (AAE, HAE and angiotensin-converting enzyme inhibitor induced angioedema) does not respond to antihistamines and corticosteroids and instead requires therapy as discussed above.