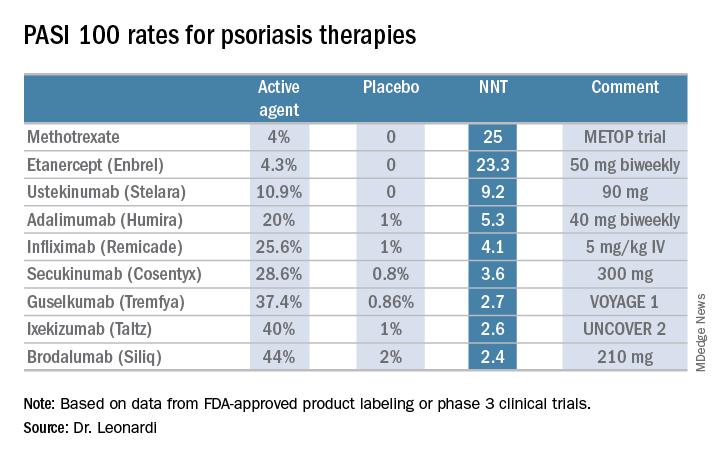
“These market leaders may not be the most relevant drugs in the current era,” according to the dermatologist.
In contrast, the high-performance biologics – the interleukin-17 inhibitors secukinumab, ixekizumab, and brodalumab and the interleukin-23 antagonist guselkumab – have impressively low NNTs of 2.4-3.6 in order to achieve complete clearance.
Turning his attention to the more modest goal of achieving a PASI 75 response, he noted that the performance of apremilast and methotrexate is “not that bad, actually, not that bad at all,” with NNTs of 3.6 and 3.2. The market leaders, adalimumab and ustekinumab, are tied with NNTs of 1.6.“But our IL-17 and IL-23 antagonists are markedly different from all other therapies, with NNTs of 1.3-1.1. With an NNT of 1.1, if you treated 11 patients with ixekizumab, 10 of them would achieve a PASI 75,” he explained.