Dermatologists are uniquely equipped amongst clinicians to make bedside diagnoses because of the focus on histopathology and microscopy inherent in our training. This skill is highly valuable in both an inpatient and outpatient setting because it may lead to a rapid diagnosis or be a useful adjunct in the initial clinical decision-making process. Although expert microscopists may be able to garner relevant information from scraping almost any type of lesion, bedside microscopy primarily is used by dermatologists in the United States for consideration of infectious etiologies of a variety of cutaneous manifestations.1,2
Basic Principles
Lesions that should be considered for bedside microscopic analysis in outpatient settings are scaly lesions, vesiculobullous lesions, inflammatory papules, and pustules1; microscopic evaluation also can be useful for myriad trichoscopic considerations.3,4 In some instances, direct visualization of the pathogen is possible (eg, cutaneous fungal infections, demodicidosis, scabetic infections), and in other circumstances reactive changes of keratinocytes or the presence of specific cell types can aid in diagnosis (eg, ballooning degeneration and multinucleation of keratinocytes in herpetic lesions, an abundance of eosinophils in erythema toxicum neonatorum). Different types of media are used to best prepare tissue based on the suspected etiology of the condition.
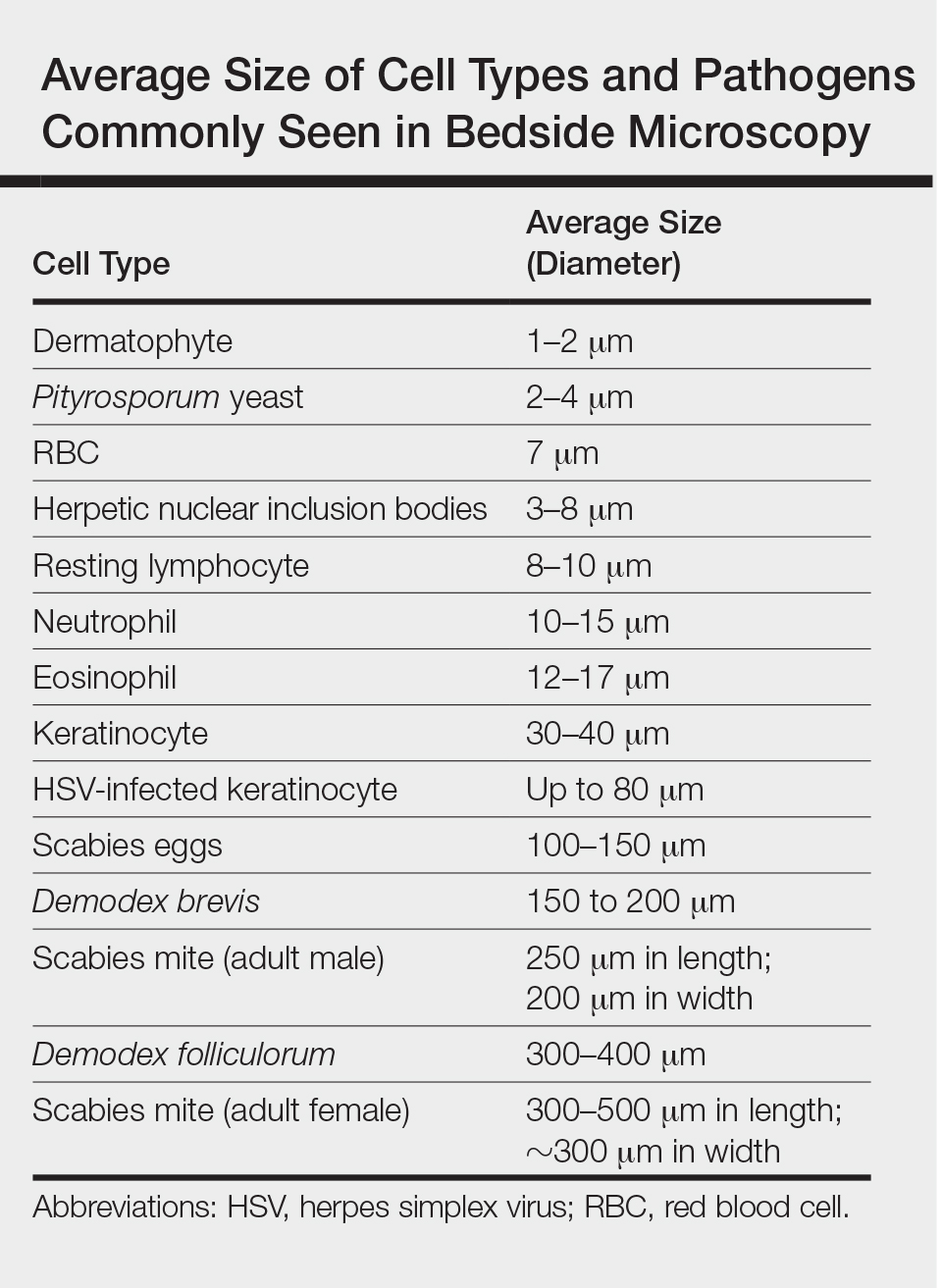
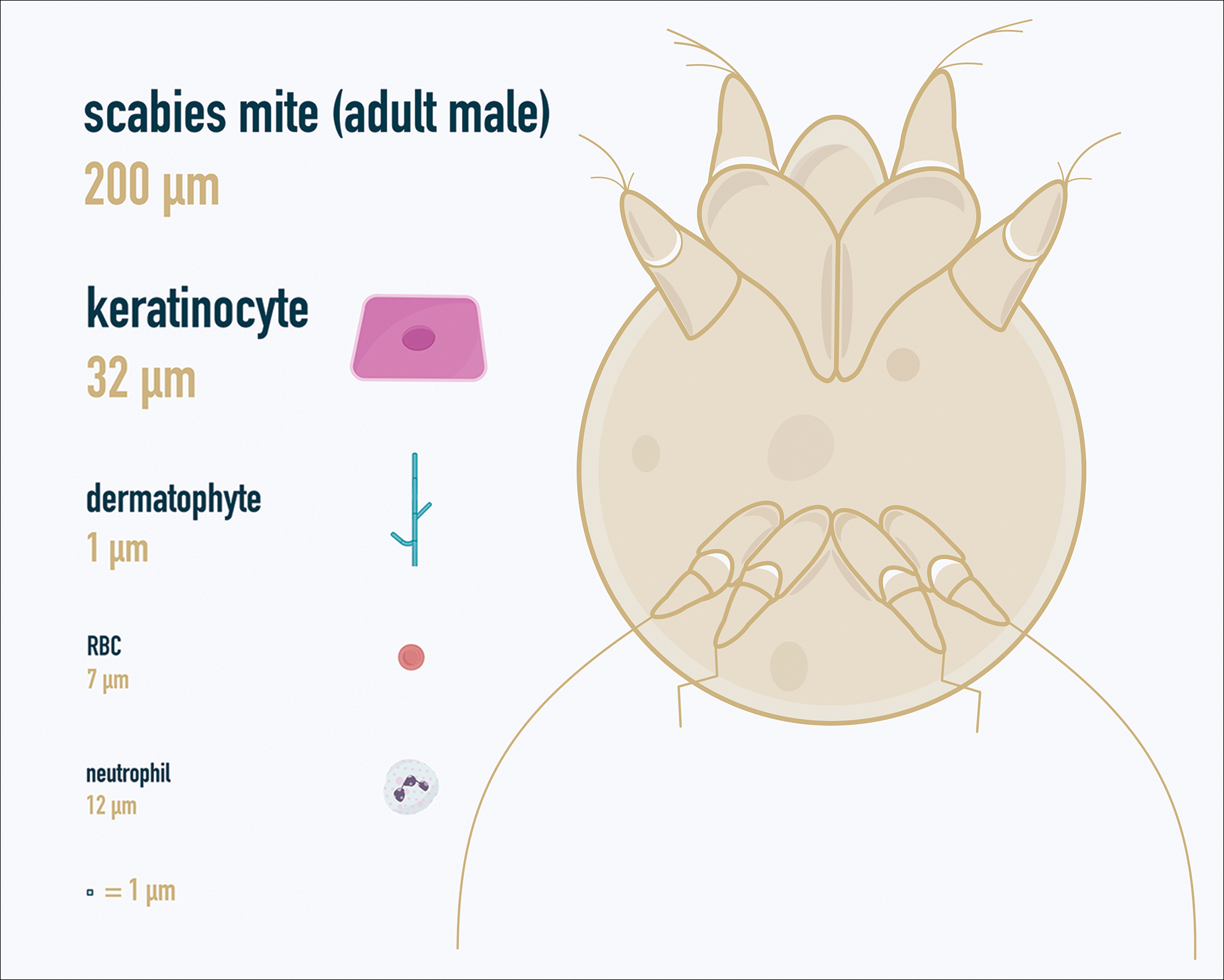
One major stumbling block for residents when beginning to perform bedside testing is the lack of dimensional understanding of the structures they are searching for; for example, medical students and residents often may mistake fibers for dermatophytes, which typically are much larger than fungal hyphae. Familiarizing oneself with the basic dimensions of different cell types or pathogens in relation to each other (Table) will help further refine the beginner’s ability to effectively search for and identify pathogenic features. This concept is further schematized in Figure 1 to help visualize scale differences.
Examination of the Specimen
Slide preparation depends on the primary lesion in consideration and will be discussed in greater detail in the following sections. Once the slide is prepared, place it on the microscope stage and adjust the condenser and light source for optimal visualization. Scan the specimen in a gridlike fashion on low power (usually ×10) and then inspect suspicious findings on higher power (×40 or higher).
Dermatomycoses
Fungal infections of the skin can present as annular papulosquamous lesions, follicular pustules or papules, bullous lesions, hypopigmented patches, and mucosal exudate or erosions, among other manifestations.5 Potassium hydroxide (KOH) is the classic medium used in preparation of lesions being assessed for evidence of fungus because it leads to lysis of keratinocytes for better visualization of fungal hyphae and spores. Other media that contain KOH and additional substrates such as dimethyl sulfoxide or chlorazol black E can be used to better highlight fungal elements.6
Dermatophytosis
Dermatophytes lead to superficial infection of the epidermis and epidermal appendages and present in a variety of ways, including site-specific infections manifesting typically as erythematous, annular or arcuate scaling (eg, tinea faciei, tinea corporis, tinea cruris, tinea manus, tinea pedis), alopecia with broken hair shafts, black dots, boggy nodules and/or scaling of the scalp (eg, tinea capitis, favus, kerion), and dystrophic nails (eg, onychomycosis).5,7 For examination of lesional skin scrapings, one can either use clear cellophane tape against the skin to remove scale, which is especially useful in the case of pediatric patients, and then press the tape against a slide prepared with several drops of a KOH-based medium to directly visualize without a coverslip, or scrape the lesion with a No. 15 blade and place the scales onto the glass slide, with further preparation as described below.8 For assessment of alopecia or dystrophic nails, scrape lesional skin with a No. 15 blade to obtain affected hair follicles and proximal subungual debris, respectively.6,9
Once the cellular debris has been obtained and placed on the slide, a coverslip can be overlaid and KOH applied laterally to be taken up across the slide by capillary action. Allow the slide to sit for at least 5 minutes before analyzing to better visualize fungal elements. Both tinea and onychomycosis will show branching septate hyphae extending across keratinocytes; a common false-positive is identifying overlapping keratinocyte edges, which are a similar size, but they can be distinguished from fungi because they do not cross multiple keratinocytes.1,8 Tinea capitis may demonstrate similar findings or may reveal hair shafts with spores contained within or surrounding it, corresponding to endothrix or ectothrix infection, respectively.5
Pityriasis Versicolor and Malassezia Folliculitis
Pityriasis versicolor presents with hypopigmented to pink, finely scaling ovoid papules, usually on the upper back, shoulders, and neck, and is caused by Malassezia furfur and other Malassezia species.5 Malassezia folliculitis also is caused by this fungus and presents with monomorphic follicular papules and pustules. Scrapings from the scaly papules will demonstrate keratinocytes with the classic “spaghetti and meatballs” fungal elements, whereas Malassezia folliculitis demonstrates only spores.5,7
Candidiasis
One possible outpatient presentation of candidiasis is oral thrush, which can exhibit white mucosal exudate or erythematous patches. A tongue blade can be used to scrape the tongue or cheek wall, with subsequent preparatory steps with application of KOH as described for dermatophytes. Cutaneous candidiasis most often develops in intertriginous regions and will exhibit erosive painful lesions with satellite pustules. In both cases, analysis of the specimen will show shorter fatter hyphal elements than seen in dermatophytosis, with pseudohyphae, blunted ends, and potentially yeast forms.5