WASHINGTON – The flu vaccine may not be perfect, but it can reduce the severity of illness and curb the risk of spreading the disease to others, William Schaffner, MD, emphasized at a press conference held by the National Foundation for Infectious Diseases.
“Give the vaccine credit for softening the blow,” said Dr. Schaffner, medical director of NFID and a professor at Vanderbilt University in Nashville.
Dr. Schaffner and a panel of experts including U.S. Surgeon General Jerome M. Adams, MD, encouraged the public and the health care community to follow recommendation from the Centers for Disease Control & Prevention that everyone aged 6 months and older receive an influenza vaccine.
Dr. Schaffner shared recent data showing that complications from the flu don’t stop when the acute illness resolves. Acute influenza causes a whole-body inflammatory reaction, and consequently “there is an increased risk of heart attack and stroke during the 2-4 weeks of recovery from acute influenza,” he said. In addition, older adults who experience acute flu and are already frail may never regain their pre-flu level of function, as the flu can start a “domino effect of decline and disability.”
Despite last year’s severe flu season that included 180 deaths in children, vaccination remains the most effective protection against the flu, Dr. Adams said.
This year, between 163 million and 168 million doses of vaccine will be available in the United States. The vaccine is available in a range of settings including doctors’ offices, pharmacies, grocery stores, and workplaces, said Dr. Adams.
Flu vaccine choices this year include a return of the live-attenuated influenza vaccine (LAIV) given via nasal spray, along with the standard influenza vaccine that includes either three influenza viruses (trivalent, with two influenza A and one influenza B) or four influenza viruses (quadrivalent, with two influenza A and two influenza B). Other options are adjuvanted vaccine and high-dose vaccine for adults aged 65 years and older, and a cell-based and recombinant vaccine as alternatives to egg-based vaccines.
Dr. Adams emphasized the importance of healthy people getting vaccinated to protect the community. “All the people who died from the flu caught it from someone else,” he said.
The message to health care providers remains the same: Recommend the flu vaccine to patients at every opportunity, and lead by example and get vaccinated yourself, Dr. Adams said. He noted this year’s strategies to promote flu vaccination on social media, and encouraged clinicians to recommend the flu shot to their patients and to showcase their own shots via the #FightFlu hashtag.
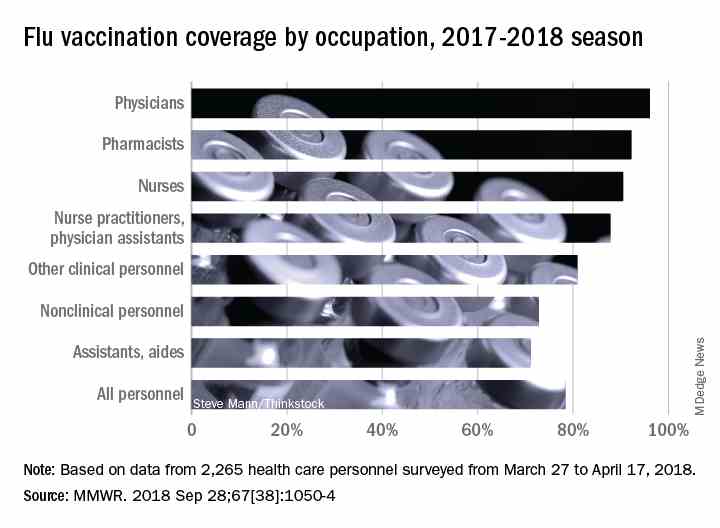
Vaccination among health care personnel last year was approximately 78%, which is a plateau over the past several years (MMWR 2018; 67:1050-54).
Be prepared to offer antivirals to patients as appropriate, and to promote the pneumococcal vaccine to eligible older adults as well, to protect not only themselves, but their contacts and the community, Dr. Adams emphasized. Currently approved antiviral drugs recommended for the 2018-2019 flu season: oseltamivir, zanamivir, and peramivir.
Wendy Sue Swanson, MD, of Seattle Children’s Hospital, stressed the importance of flu vaccination for all children, given their ability to spread viral infections. She noted a concerning 2% drop in vaccinations for children aged 6 months to 4 years, although vaccination coverage in this group was highest among children overall, at approximately 68% last season.
Last year, approximately 80% of the child deaths from flu occurred in unvaccinated children, but the vaccine has been shown to reduce the likelihood of hospitalization or death even if a child does become ill, Dr. Swanson said.
Laura E. Riley, MD, of Weill Cornell Medical Center, noted that vaccination of pregnant women has plateaued in recent years, and was 49% last year. “Our goal is 80% plus,” she said. Data show that pregnant women who received flu vaccination were 40% less likely to be hospitalized for the flu, she noted. The American College of Obstetricians and Gynecologists recommends flu vaccination as safe during any trimester, and valuable to both mothers and newborns because it provides protective antibodies during the first 6 months of life before babies can receive their own vaccinations, Dr. Riley said.
More information about this year’s flu season is available from the CDC and NFID.