Comment
BRAF Inhibitors
Normally, activated BRAF phosphorylates and stimulates MEK proteins, ultimately influencing cell proliferation, survival, and differentiation.3-5 BRAF mutations that constitutively activate this pathway have been detected in several malignancies, including papillary thyroid cancer, colorectal cancer, and brain tumors, but they are particularly prevalent in melanoma.4,6 The majority of BRAF-positive malignant melanomas are associated with V600E, in which valine is substituted for glutamic acid at codon 600. The next most common BRAF mutation is V600K, in which valine is substituted for lysine.2,7 Together these constitute approximately 95% of BRAF mutations in melanoma patients.5
MEK Inhibitors
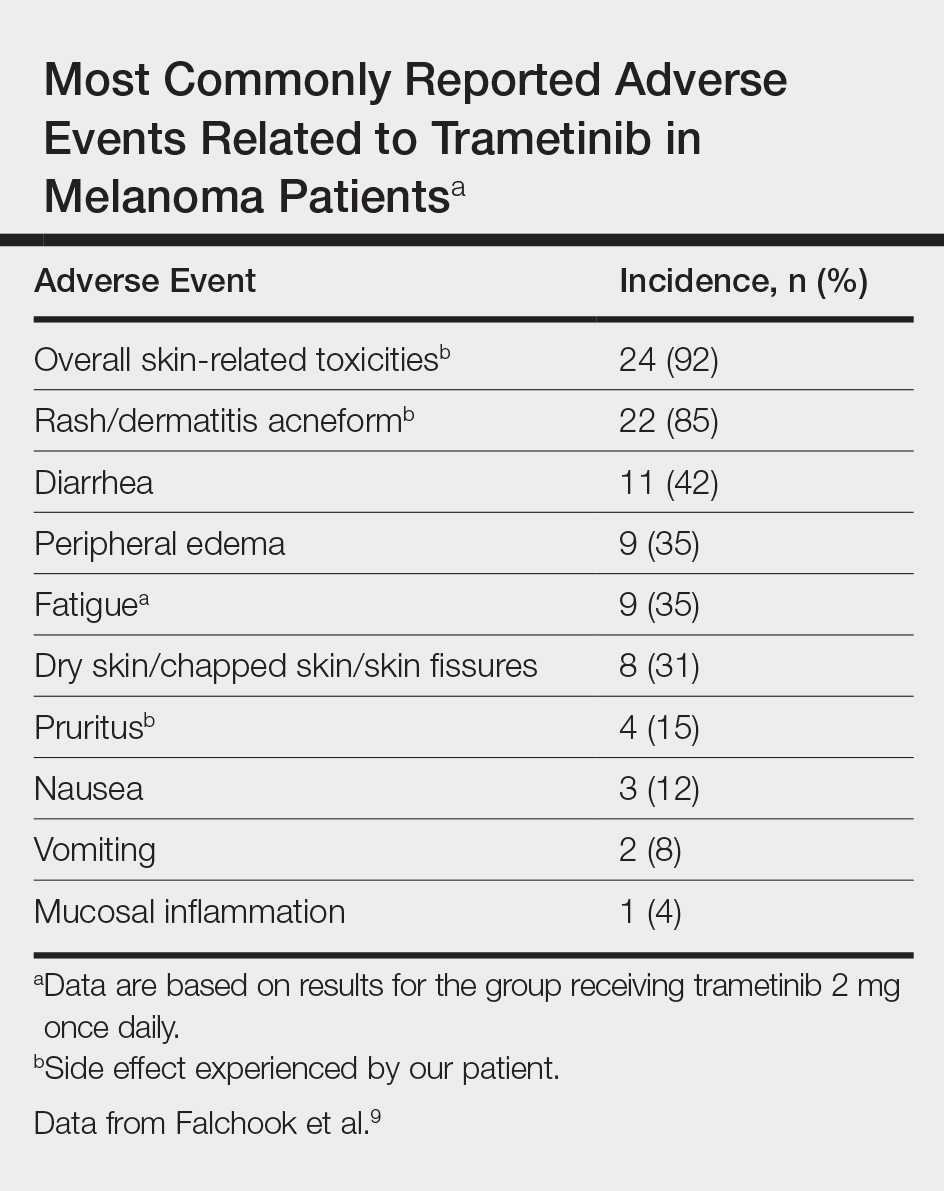
Initially, BRAF inhibitors (BRAFi) were introduced to the market for treating melanoma with great success; however, resistance to BRAFi therapy quickly was identified within months of initiating therapy, leading to investigations for combination therapy with MEK inhibitors (MEKi).2,5 MEK inhibition decreases cellular proliferation and also leads to apoptosis of melanoma cells in patients with BRAF V600E or V600K mutations.2,8 Trametinib, in particular, is a reversible, highly selective allosteric inhibitor of both MEK1 and MEK2. While on trametinib, patients with metastatic melanoma have experienced 3 times as long progression-free survival as well as 81% overall survival compared to 67% overall survival at 6 months in patients on chemotherapy, dacarbazine, or paclitaxel.5 However, AEs are quite common with trametinib, with cutaneous AEs being a leading side effect. Several large trials have reported that 57% to 92% of patients on trametinib report cutaneous AEs, with the majority of cases being described as papulopustular or acneform (Table).5,9
Combination Therapy
Fortunately, combination treatment with a BRAFi may alleviate MEKi-induced cutaneous drug reactions. In one study, acneform eruptions were identified in only 10% of those on combination therapy—trametinib with the BRAFi dabrafenib—compared to 77% of patients on trametinib monotherapy.10 Strikingly, cutaneous AEs occurred in 100% of trametinib-treated mice compared to 30% of combination-treated mice in another study, while the benefits of MEKi remained similar in both groups.11 Because BRAFi and MEKi combination therapy improves progression-free survival while minimizing AEs, we support the use of combination therapy instead of BRAFi or MEKi monotherapy.5
Histologic Evidence of AEs
Histology of trametinib-associated cutaneous reactions is not well characterized, which is in contrast to our understanding of cutaneous AEs associated with BRAFi in which transient acantholytic dermatosis (seen in 45% of patients) and verrucal keratosis (seen in 18% of patients) have been well characterized on histology.12 Interestingly, cutaneous granulomatous eruptions have been attributed to BRAFi therapy in 4 patients.13,14 One patient was on monotherapy with vemurafenib and granulomatous dermatitis with focal necrosis was seen on histology.13 The other 3 patients were on combination therapy with trametinib; 2 had histology-proven sarcoidal granulomatous inflammation, and 1 demonstrated perifollicular granulomatous inflammation and granulomatous inflammation surrounding a focus of melanoma cells.13,14 Although these granulomatous reactions were attributed to BRAFi or combination therapy, the association with trametinib remains unclear. On the other hand, our patient’s granulomatous reaction was exacerbated on trametinib monotherapy, suggesting a relationship to trametinib itself rather than BRAFi.
Conclusion
With the discovery of molecular targeting in melanoma, BRAFi and MEKi therapies provide major milestones in metastatic melanoma management. As more patients are treated with these agents, it is important that we better characterize their associated side effects. Our case of an unusual xanthogranulomatous reaction to trametinib adds to the knowledge base of possible cutaneous reactions caused by this drug. We hope that prospective studies will further investigate and differentiate the cutaneous AEs described so that we can better manage these patients.