Cantharidin, used for decades by pediatric dermatologists to treat molluscum contagiosum, has become hard to obtain, according to several dermatologists and a supplier.
Physicians suspect, and a Food and Drug Administration document suggests, the FDA is blocking importation of the agent.
Cantharidin has been a concern at the FDA since at least the late 1990s, when it was proposed for but not added to the list of bulk substances allowed by the agency to be compounded. The substance has never gone through formal agency review and approval.
According to Deborah Grafelman, president of Delasco Dermatologic Lab and Supply in Council Bluffs, Iowa, it was only recently that cantharidin imports from China have been blocked.
Her company once supplied cantharidin to about 700 clients, including physician offices, clinics, and hospitals, at a volume of about 2,500 vial sales per year, but this activity has stopped.
The FDA has "apparently been cracking down on it recently. We could get cantharidin from our supplier in 2008," but not since, she said.
Dr. Robert Sidbury, chief of the division of dermatology at Seattle Children's Hospital and the University of Washington, said he had been told by FDA staff that they encourage interested physicians to request an Investigational New Drug (IND) application, which would allow use of cantharidin as an investigational drug.
There was talk of doing just that at the Society for Pediatric Dermatology’s annual meeting in Portland, Ore., this summer.
Following a presentation by Dr. Sidbury that touched upon the situation, an audience member said "we need to take collective action and work together, not play victim. As subspecialists, we can prove it's in the best interests of patients ... even if it takes years to get an IND and do a study to show it's safe."
After repeated inquiries from Skin & Allergy News, the FDA did not provide any information on the situation, including how many shipments may have been blocked and if the agency has new safety concerns about cantharidin. Agency officials declined to comment.
However, a Jan. 7, 1999, Federal Register notice that proposed cantharidin for the bulk-substance list gave insight into the agency's concerns. It refers to cantharidin as an "an extremely toxic substance."
An Aug. 31, 2009, order blocking Delasco's importation of 0.1 kg noted, "The article appears to be a new drug without an approved new drug application."
The same issue was raised in an FDA Jan. 14, 2008, warning letter to Bellevue Pharmacy Solutions of St. Louis, Mo., which ordered the pharmacy to stop compounding cantharidin.
Because cantharidin is not an active ingredient in any FDA-approved drug, the "FDA does not sanction" its use in pharmacy compounding, the letter noted.
The agency "will not exercise enforcement discretion toward your firm's continued compounding of" cantharidin, however, failure to comply could result in "legal action without further notice, including, without limitation, seizure and injunction," according to the letter.
Officials from Bellevue Pharmacy Solutions declined to comment for this story.
Dermatologists are frustrated that a treatment they have relied on for years is no longer available.
"Used appropriately, it's effective and well tolerated," said Dr. Anthony J. Mancini, head of the division of pediatric dermatology at Northwestern University and Children's Memorial Hospital in Chicago, who used to purchase cantharidin from Delasco Dermatologic Lab and Supply.
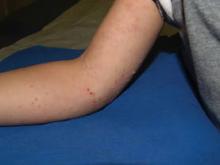
"It's the best treatment for molluscum, successful in over 95% of our patients. We are just trying to offer patients what’s best for them, and now we can't even apply a little beetle juice to these common and often irritating lesions," Dr. Mancini said.
Last winter, Dr. Mancini and his colleagues discovered they could no longer get cantharidin, and became aware of the FDA's apparent ban on the product.
In a March 15 notice to "all faculty, trainees, [and] nurses in dermatology," he wrote, "as of today, we are no longer allowed to utilize cantharidin from any compounding pharmacy to treat molluscum."
Also on March 15, he asked the agency by e-mail "if it is possible to convene an FDA group to re-evaluate this agent."
The message included five references to studies over the past decade that show cantharidin to be a safe and effective molluscum treatment. Dr. Mancini said he has received no response to his message.
Cantharidin is a vesicant extracted from crushed Chinese blister beetles. Applied topically, it is absorbed into epidermal cell membranes, leading to acantholysis, intraepidermal blistering, and resolution of the lesion. Redness and transient burning sensations are among possible complications, though these effects are exceedingly rare. Use of the agent generally does not lead to scarring. However, severe blistering can occur with improper use; and ingestion, especially by children, can be fatal (Arch. Dermatol. 2001;137:1357-60).