To the Editor:
A 58-year-old woman with a history of hyperprolactinemia and gastrointestinal angiodysplasia presented to the dermatology department with a generalized skin rash of 3 weeks’ duration. She did not have a history of toxic habits. She had a history of chronic hepatitis C virus (HCV) genotype 1b (IL-28B locus) with severe hepatic fibrosis (stage 4) as assessed by ultrasound-based elastography. Due to lack of response, plasma HCV RNA was still detectable at week 12 of pegylated interferon and ribavirin (RIB) therapy, and triple therapy with pegylated interferon, RIB, and telaprevir was initiated.
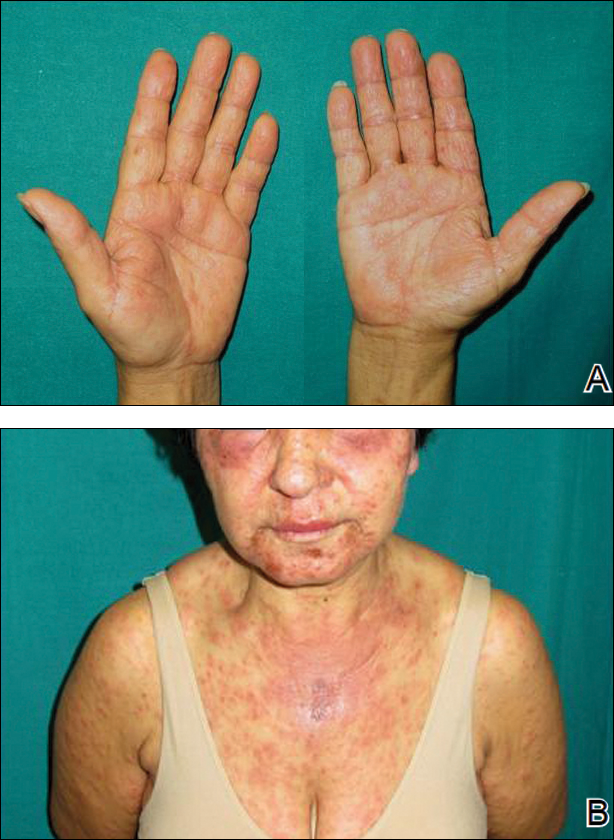
Two months later, she was admitted to the hospital after developing a generalized cutaneous rash that covered 90% of the body surface area (BSA) along with fever (temperature, 38.5°C). Laboratory blood tests showed an elevated absolute eosinophil count (2000 cells/µL [reference range, 0–500 cells/µL]), anemia (hemoglobin, 6.5 g/dL [reference range, 12–16 g/dL]), thrombocytopenia (26×103/µL [reference range, 150–400×103/µL]), and altered liver function tests (serum alanine aminotransferase, 60 U/L [reference range, 0–45 U/L]; aspartate aminotransferase, 80 U/L [reference range, 0–40 U/L]). Plasma HCV RNA was undetectable at this visit. On physical examination a generalized exanthema with coalescing plaques was observed, as well as crusted vesicles covering the arms, legs, chest, abdomen, and back. Palmoplantar papules (Figure, A) and facial swelling (Figure, B) also were present. A skin biopsy specimen taken from a papule on the left arm showed superficial perivascular lymphocytic infiltration with dermal edema. These findings were consistent with a diagnosis of DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome. Application of the Adverse Drug Reaction Probability Scale1 in our patient (total score of 5) suggested that DRESS syndrome was a moderate adverse event likely related to the use of telaprevir.
After diagnosis of DRESS syndrome, telaprevir was discontinued, and the doses of RIB and pegylated interferon were reduced to 200 mg and 180 µg weekly, respectively. Laboratory test values including liver function tests normalized within 3 weeks and remained normal on follow-up. Plasma HCV RNA continued to be undetectable.
Hepatitis C virus is relatively common with an incidence of 3% worldwide.2 It may present as an acute hepatitis or, more frequently, as asymptomatic chronic hepatitis. The acute process is self-limited and rarely causes hepatic failure. It usually leads to a chronic infection, which can result in cirrhosis, hepatocellular carcinoma, and the need for liver transplantation. The aim of treatment is eradication of HCV RNA, which is predicted by the attainment of a sustained virologic response. The latter is defined by the absence of HCV RNA by a polymerase chain reaction within 3 to 6 months after cessation of treatment.
Treatment of chronic HCV was based on the combination of pegylated interferon alfa-2a or -2b with RIB until 2015. Guidelines for the diagnosis and management of HCV infection have been published by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.2 These guidelines include new protease inhibitors, telaprevir and boceprevir, in the therapeutic approach of these patients. The main limitation of both drugs is the cutaneous toxicity.
Factors to be considered when treating HCV include viral genotype, if the patient is naïve or pretreated, the degree of fibrosis, established cirrhosis, and the treatment response. For patients with genotype 1,2 as in our case, combination therapy with 3 drugs is recommended: pegylated interferon 180 µg subcutaneous injection weekly, RIB 15 mg/kg daily, and telaprevir 2250 mg or boceprevir 2400 mg daily. Triple therapy has been shown to achieve a successful response in 75% of naïve patients and in 50% of patients refractory to standard therapy.3
Telaprevir is an NS3/4A protease inhibitor approved by the US Food and Drug Administration and the European Medicines Agency for treatment of chronic HCV infection in naïve patients and in those unresponsive to double therapy. In phase 2 clinical trials, 41% to 61% of patients treated with telaprevir developed cutaneous reactions, of which 5% to 8% required cessation of treatment.4 The predicting risk factors for developing a secondary rash to telaprevir include age older than 45 years, body mass index less than 30, Caucasian ethnicity, and receiving HCV therapy for the first time.4
This cutaneous side effect is managed depending on the extension of the lesions, the presence of systemic symptoms, and laboratory abnormalities.5 Therefore, the severity of the skin reaction can be divided into 4 stages4,5: (1) grade I or mild, defined as a localized rash with no systemic signs or mucosal involvement; (2) grade II or moderate, a maximum of 50% BSA involvement without epidermal detachment, and inflammation of the mucous membranes may be present without ulcers, as well as systemic symptoms such as fever, arthralgia, or eosinophilia; (3) grade III or severe, skin lesions affecting more than 50% BSA or less if any of the following lesions are present: vesicles or blisters, ulcers, epidermal detachment, palpable purpura, or erythema that does not blanch under pressure; (4) grade IV or life-threatening, when the clinical picture is consistent with acute generalized exanthematous pustulosis, DRESS syndrome, toxic epidermal necrolysis, or Stevens-Johnson syndrome.
DRESS syndrome is a condition clinically characterized by a generalized skin rash, facial angioedema, high fever, lymph node enlargement, and leukocytosis with eosinophilia or atypical lymphocytosis, along with abnormal renal and hepatic function tests. Cutaneous histopathologic examination may be unspecific, though atypical lymphocytes with a marked epidermotropism mimicking fungoid mycosis also have been described.6 In addition, human herpesvirus 6 serology may be negative, despite infection with this herpesvirus subtype having been associated with the development of DRESS syndrome. The pathophysiologic mechanism of DRESS syndrome is not completely understood; however, one theory ascribes an immunologic activation due to drug metabolite formation as the main mechanism.1
Eleven patients7 with possible DRESS syndrome have been reported in clinical trials (less than 5% of the total of patients), with an addition of 1 more by Montaudié et al.8 No notable differences were found between telaprevir levels in these patients with respect to those of the control group.
For the management of DRESS syndrome, the occurrence of early signs of a severe acute skin reaction requires the immediate cessation of the drug, telaprevir in this case. The withdrawal of the dual therapy will depend on the short-term clinical course, according to the general condition of the patient, as well as the analytical abnormalities observed.9
In conclusion, telaprevir is a promising novel therapy for the treatment of HCV infection, but its cutaneous side effects still need to be properly established.