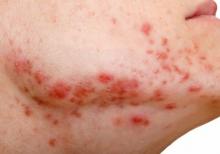
There is when based on reductions in inflammatory lesion counts, according to a recent Cochrane Review.
“The current recommendation of clinical guidelines that oral isotretinoin should be the first-line treatment for moderate to severe acne unresponsive to previous therapies, being more effective than the use of oral antibiotics plus topical agents, underpins current dermatological practice,” Caroline S. Costa, MD, from the Emergency Medicine and Evidence-Based Medicine department at Universidade Federal de São Paulo and her colleagues wrote in their review. “The recommendation cannot be fully supported with certainty by the finding of this review; however, neither does this review challenge it.”
Dr. Costa and her colleagues identified 31 randomized, controlled trials in the Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase, PsycINFO, and LILACS databases up to July 2017, which altogether included 3,836 participants with mild to severe acne who were aged 12-55 years. The patients received treatment with oral isotretinoin versus placebo or other treatments, such as antibiotics.
In 3 studies that altogether included 400 participants and compared isotretinoin with oral antibiotics plus topical treatments, there was no significant difference with isotretinoin in decreases in the investigator-assessed inflammatory lesion count in participants with moderate to severe acne after 20-24 weeks (risk ratio, 1.01; 95% confidence interval, 0.96-1.06). One serious side effect – Stevens-Johnson syndrome – was noted in the isotretinoin-treated group (RR, 3.00; 95% CI, 0.12-72.98). These results were based on “very-low-quality evidence,” the authors noted.
Two studies also comparing isotretinoin with oral antibiotics and topical treatments in a total of 351 participants showed a 15% improvement in acne severity (RR, 1.15; 95% CI, 1.00-1.32) as assessed by physician’s global evaluation but with a greater number of adverse events considered less severe (RR, 1.67; 95% CI, 1.42-1.98), such as vomiting, nausea, dry lips and skin, and cheilitis.
With regard to dosing, one study with 154 participants with severe acne showed 0.05 mg/kg per day of isotretinoin reduced inflammatory lesion count by 79% after 20 weeks, while 0.1 mg/kg per day and 0.2 mg/kg per day reduced inflammatory lesion counts by 80% and 84%, respectively, after 20 weeks. A different study of 150 participants with severe acne found a 95% decrease in inflammatory lesion counts for 58% of participants receiving 0.1 mg/kg per day, 80% of participants receiving 0.5 mg/kg per day, and 90% of participants receiving 1 mg/kg per day of oral isotretinoin after 20 weeks.
In a randomized, controlled trial with 40 participants with moderate acne comparing intermittent dosing (0.5-0.7 mg/kg per day for 1 week per month), continuous conventional dosing (0.5-0.7 mg/kg per day), and continuous low dosing (0.25-0.4 mg/kg per day) of oral isotretinoin found greater differences in mean decreased numbers of inflammatory lesions among participants in the continuous low dose (a decrease of 3.72 lesions; 95% CI, 2.13-5.31) and continuous conventional dose (a decrease of 3.87 lesions; 95% CI, 2.31-5.43) groups. The authors noted they were unable to perform a meta-analysis because of study heterogeneity in the three studies examining the primary outcome.
In 14 studies with a total of 906 participants who had moderate and severe acne, the authors noted there were no severe adverse events with different doses and regimens of isotretinoin treatment from 12 weeks to 32 weeks or at follow-up at the end of treatment at up to 48 weeks. There were some adverse events, such as skin dryness, hair loss, and itching in 13 studies with a total of 858 participants.
No birth defects were reported in the studies.