Background
Merkel cell carcinoma (MCC) is a rare, aggressive cutaneous malignancy.1 Immunosuppression, advanced age, and UV light exposure of fair skin are major risk factors; additionally, polyomavirus infection is detected in as many as 80% of cases.2-5 Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis.6 Approximately half of patients present with localized disease, in which surgical resection with or without adjuvant radiotherapy is usually indicated. However, overall prognosis is poor with MCC due to high recurrence rates.3
This neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes; distant metastasis also can occur, most commonly to the liver, lungs, bones, and brain.7,8 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. The frequency of metastasis at diagnosis as well as high recurrence rates after treatment contribute to the overall poor prognosis of MCC. Local recurrence rates have been reported at 25%, with lymph node involvement in 52% and metastasis in 34% of cases; most recurrences occur within 2 years of diagnosis.8 The aggressiveness of the tumor determines patient mortality; in cases without lymph node involvement, the 5-year survival rate is 83.3%. However, the 5-year survival rate drops to 58.3% with lymph node involvement; in patients with metastatic disease, it is just 31.3%.8,9 Although MCC is a chemosensitive disease, durable responses are rarely seen in the advanced setting.3 Most patients with metastatic disease have a median progression-free survival of only 3 months.1
Presentation and Diagnosis
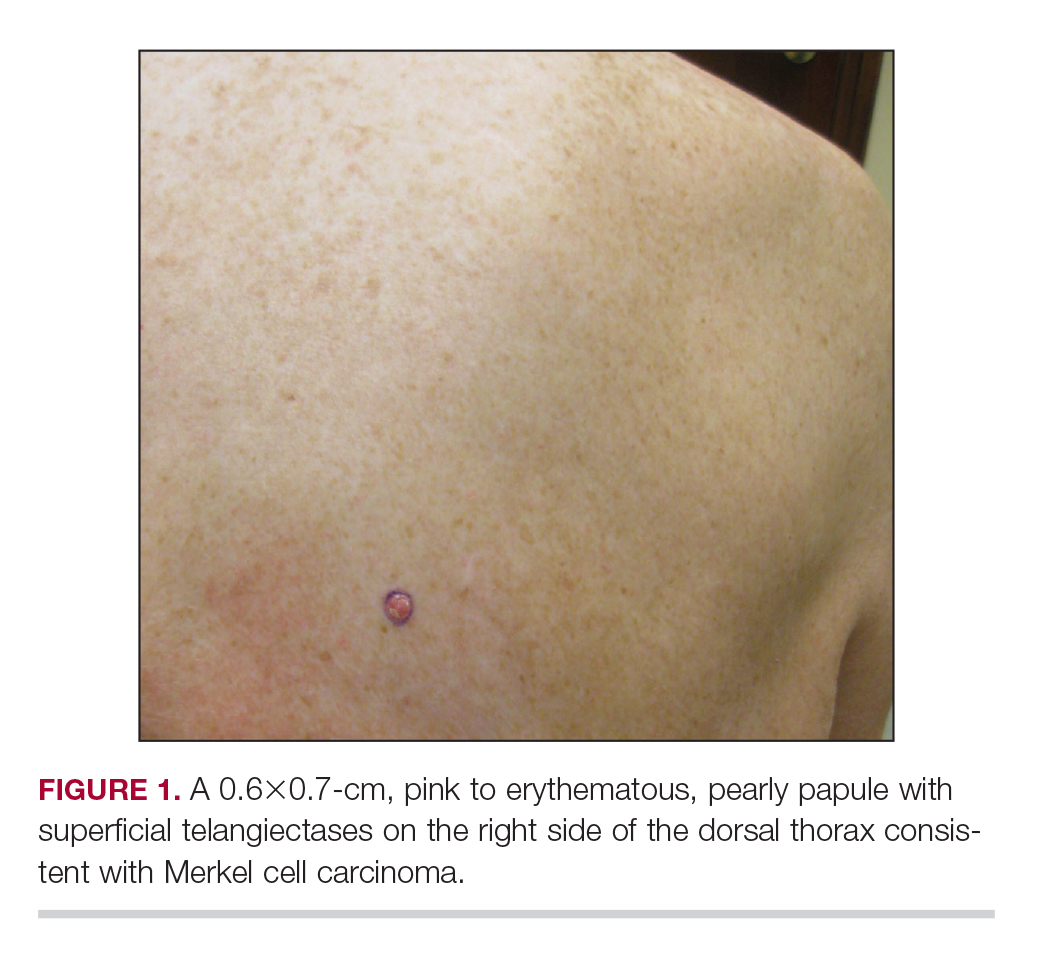
An MCC tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface, most often on the sun-exposed head and neck region.3,9-11 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% present on the extremities, and 12% to 14% on the trunk.6 Unfortunately, this nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC can only be achieved with a skin biopsy, which allows for distinction from other clinically similar-appearing neoplasms.
Specific guidelines for an MCC diagnostic evaluation have been proposed by the National Comprehensive Cancer Network (NCCN) based on a framework of clinical presentation, preliminary workup, diagnosis, and additional workup.12 The simplified algorithm for evaluation includes the following12,13:
- Examine skin and lymph nodes
- Obtain a biopsy specimen stained with hematoxylin and eosin (including Breslow thickness and evidence of lymphovascular invasion) and immunostaining (including but not limited to cytokeratin (CK) 20 and thyroid transcription factor 1)
- Obtain a sentinel lymph node biopsy specimen from patients with negative clinical nodes, prior to excision when possible
- Perform fine-needle aspiration or core biopsy first for patients with positive clinical nodes; if negative, consider open biopsy, but if positive, proceed to next step
- Perform imaging as clinically indicated with magnetic resonance imaging, computed tomography, or positron emission tomography–computed tomography
- Consider consultation with a multidisciplinary tumor board
Histologic Features of MCC
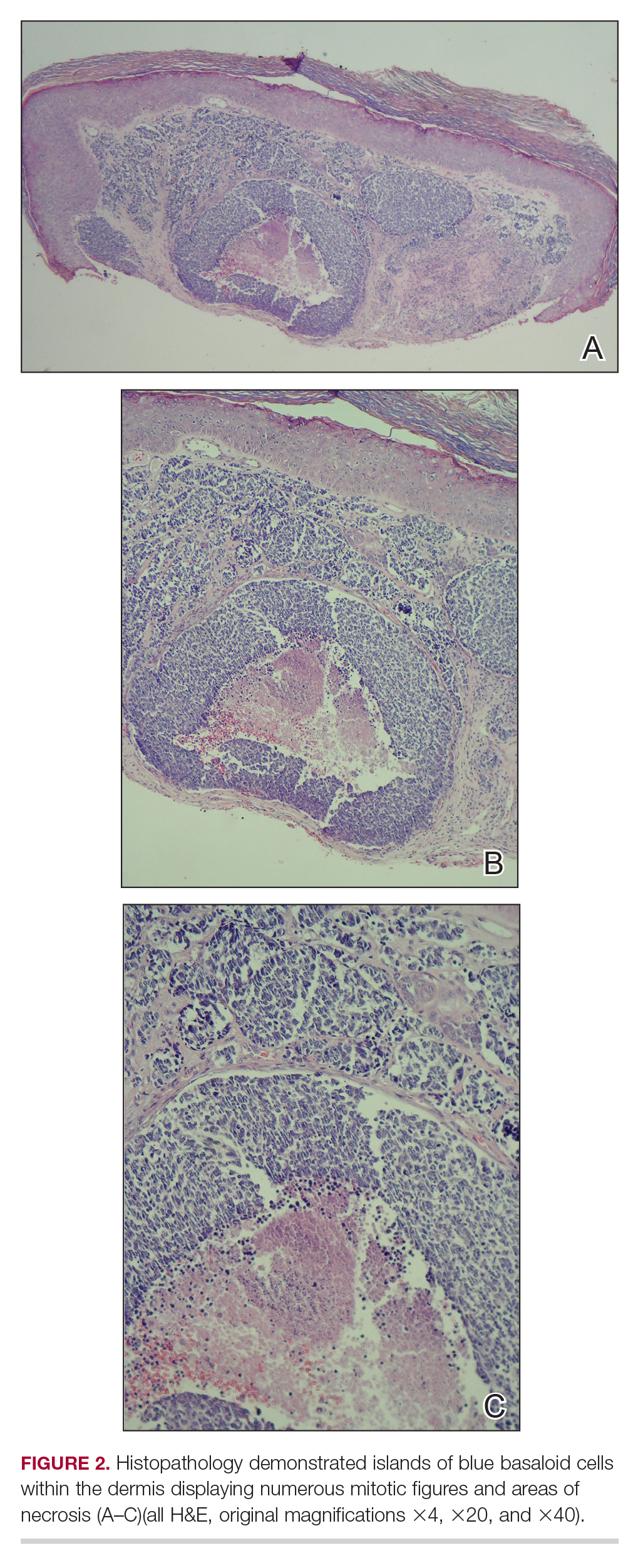
Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.8,10 Immunohistochemical features include CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and CK7 negativity, which allows it to be differentiated from other neoplasms. Chromogranin and synaptophysin positivity provide further histologic confirmation. The absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.4,8
Merkel Cell Polyomavirus and Other Risk Factors
Merkel cell carcinoma is frequently associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases.8 Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.10
Immunosuppression and UV-exposed fair skin also are considered major risk factors, which may be explained by the increase in MCPyV small T-antigen transcripts induced by UV irradiation.10 Moreover, as is seen in other cancers induced by viruses, host immunity can hinder tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development as well as the potential for a worse prognosis.4,8 The precise incidence of MCC in immunosuppressed patients appears unclear; it is possible that chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.4,8
Impact of Comorbidities on MCC
These risk factors were all observed in the case patient. However, it also is possible that his associated comorbidities also had an impact on the presentation of his disease.8 For example, patients with rheumatoid arthritis have been shown to have an elevated risk for the development of MCC.14 Moreover, inflammatory monocytes infected with MCPyV, as would be expected in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by migrating to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.8,15 Testing for MCPyV was never performed in the case patient; however, it certainly would be prudent to do so in such a presentation. Additionally, further studies to determine the correlation of MCC to these disease processes are warranted.8
Given that MCC tends to affect an older population in which other notable comorbidities are not uncommon, the cost, side effects, and convenience for patients of any treatment plan are important considerations.8 In the case study, a combined regimen of carboplatin and VP-16 (etoposide) was utilized. This regimen was well tolerated by the patient and successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination has been shown to prolong survival in patients with distant metastasis compared to those patients not receiving chemotherapy.6 In all high-risk patients, close clinical monitoring is essential to help optimize outcomes.
Bottom Line
Merkel cell carcinoma is a rare but aggressive cutaneous neoplasm that most frequently affects elderly patients, immunosuppressed patients, and individuals with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases such as psoriasis and rheumatoid arthritis also may play a role. In this case, the patient’s history of chronic immunosuppressive therapy for treatment of psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor. This potential risk should be an important point of discussion with any patient requiring this type of long-term management for disease control. This unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission following treatment with surgery and chemotherapy.