Antivenom Therapy
Crotalidae polyvalent immune fab is an antivenom comprised of purified, sheep-derived fab IgG fragments and was approved by the US Food and Drug Administration in 2000 for the treatment of North American crotalid envenomation.12,13 Its venom-specific fab fragments of IgG bind to and neutralize venom toxins and facilitate their elimination. Crotalidae polyvalent immune fab does not contain the Fc fragment of the IgG antibody, resulting in a low incidence of hypersensitivity reactions (8%) and serum sickness (13%).13 It is produced using 4 North American venoms: Crotalus atrox (western diamondback rattlesnake), Crotalus adamanteus (eastern diamondback rattlesnake), Crotalus scutulatus (Mojave rattlesnake), and Agkistrodon piscivorus (water moccasin).12 It has become the standard-of-care antivenom for pit viper bites and has replaced its equine-derived predecessor antivenin crotalidae polyvalent, which is known for high rates of acute allergic reactions (23%–56%) including anaphylaxis and delayed serum sickness.13,14 In postmarketing studies, CPIF has demonstrated control of envenomation regardless of severity. The most common adverse reactions reported include urticaria, rash, nausea, pruritus, and back pain.13 Anaphylaxis and anaphylactoid reactions may occur, and patients should be carefully observed during antivenom infusion. Appropriate management should be readily available including epinephrine, intravenous antihistamines, and/or albuterol. Contraindications include known hypersensitivity to papaya or papain, which is used to cleave the antibodies into fragments during the processing of CPIF. Patients also may react to it if allergic to other papaya extracts, chymopapain, or bromelain (pineapple enzyme), as well as some dust mite allergens and latex allergens that share antigenic structures with papain.15 Each vial of CPIF is reconstituted with 18 mL of 0.9% sodium chloride, as described in the package insert.13 The total dose (minimum of 4 to maximum of 12 vials initial dose) is then diluted in 250 mL of normal saline and infused over 1 hour starting for the first 10 minutes at a rate of 25 to 50 mL/h, and if tolerated, then increased to 250 mL/h until completion.
Treatment Algorithm
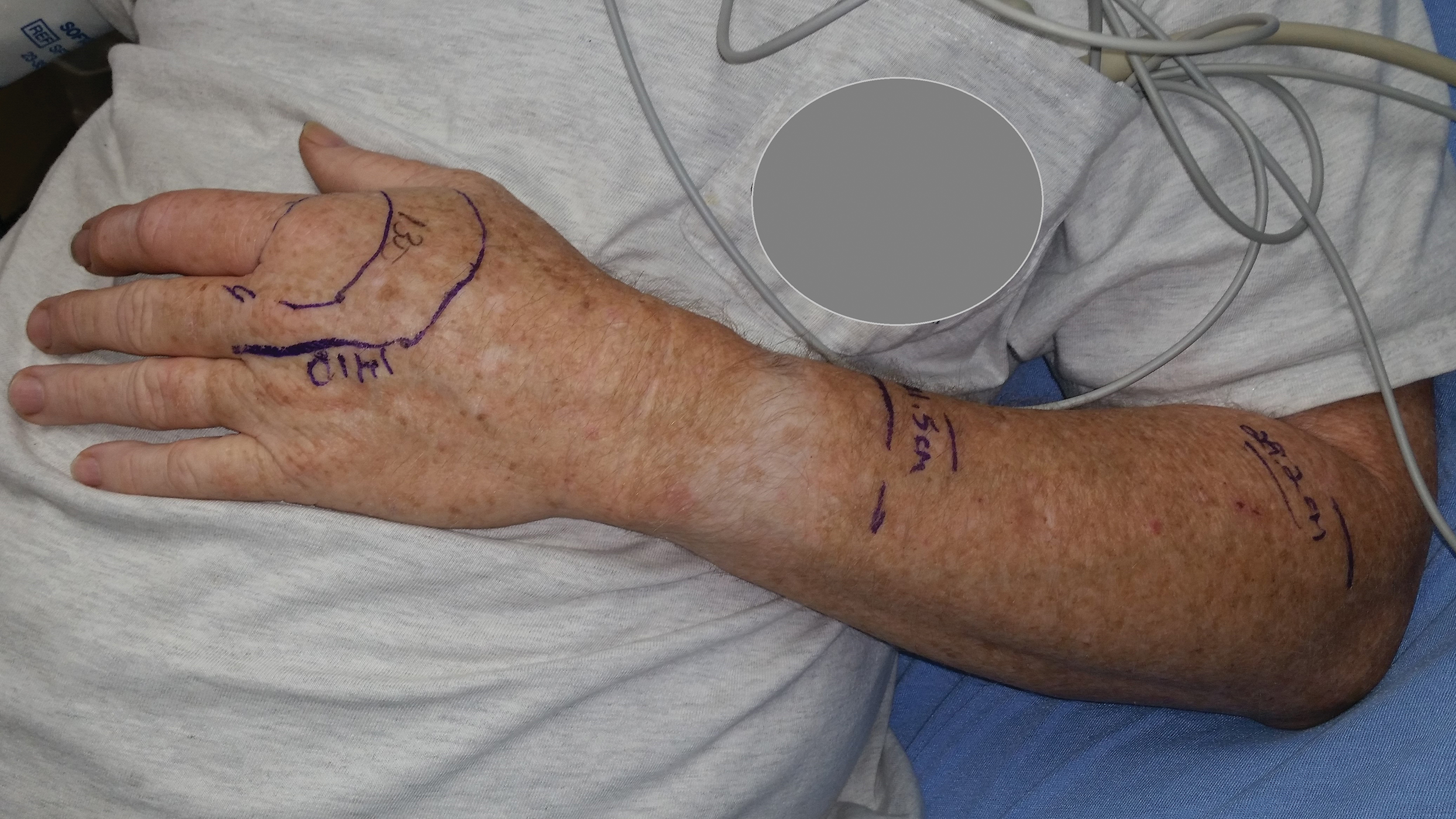
According to the envenomation consensus treatment algorithm, assess the site of the snakebite and mark leading edge of swelling every 15 to 30 minutes,16 as shown in our patient in Figure 6.Immobilize and elevate the affected extremity. Update tetanus vaccine and order initial laboratory tests to include prothrombin time, hemoglobin, platelets, and fibrinogen. If the patient does not exhibit local signs of envenomation such as redness, swelling, or ecchymosis, and the patient has no coagulation laboratory abnormalities and exhibits no systemic signs such as diarrhea, vomiting or angioedema, withhold CPIF and observe the patient for a minimum of 8 hours. Repeat laboratory tests prior to discharge. This clinical scenario most likely occurs in the setting of a dry bite or no bite at all. For minor envenomation, it also is possible to withhold CPIF and observe the patient for 12 to 24 hours if he/she remains stable and laboratory tests remain within reference range. If the patient has local or systemic signs of envenomation, start CPIF (4–6 vials and up to a maximum of 12 vials). The first dose of CPIF should be administered in the emergency department or intensive care unit. If after the first hour envenomation is worsening, an additional 4 to 6 vials may be infused. If after the first hour of observation the envenomation is controlled based on decreased swelling or lack of progression and there is improvement in laboratory values, then a maintenance regimen can be initiated. The maintenance dose consists of 2 vials every 6 hours for up to 18 hours (3 separate 2-vial doses). Patients can be discharged if stable and with no negative laboratory trends during the observation period.16 If CPIF was administered, follow-up laboratory tests results are dependent on prior findings of coagulation abnormalities, degree of envenomation, and signs and symptoms of coagulopathy postdischarge. Recurrent coagulopathy can occur in patients with coagulation abnormalities during initial envenomation, and patients should be monitored for possible re-treatment for at least 1 week or longer.17 Coagulopathy can present with decreased fibrinogen, decreased platelets, and elevated prothrombin time. Our patient experienced a drop in platelet count and hemoglobin level in addition to localized tissue effects, but he responded to the antivenom therapy. Lastly and importantly, no pediatric adjustments are necessary, and although mercury has been removed from the product’s manufacturing process, certain easily identifiable antivenom lots that have not expired contain ethyl mercury from thimerosal.13,18,19 Some side effects of thimerosal include redness and swelling of the injection site, but scientific research does not show a connection with autism.20
Conclusion
Dusky pigmy rattlesnake envenomations are clinically responsive to CPIF antivenom treatment.21 Although no clearly documented fatalities have been reported from dusky pigmy rattlesnake bites, coagulopathy and local tissue necrosis—as described in our patient—can result from such snakebites, requiring hospitalization. These snakes are common in the southeastern United States, and the treatment algorithm presented can be extrapolated to other more serious and deadly pit viper bites.