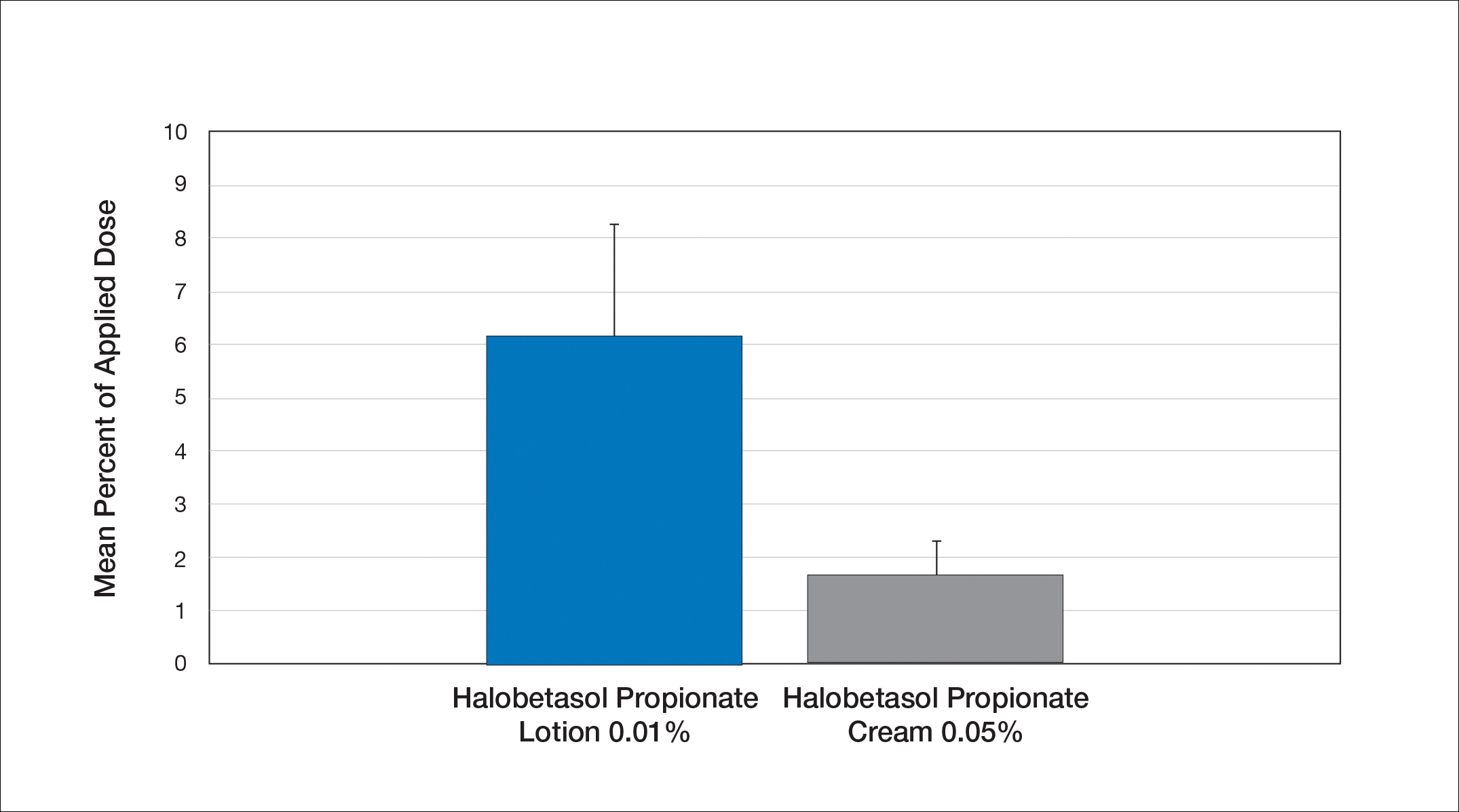
Comparison of Skin Penetration of HP Lotion 0.01% vs HP Cream 0.05%
Comparative percutaneous absorption of 2 HP formulations—0.01% lotion and 0.05% cream—was evaluated in vitro using human tissue from a single donor mounted on Bronaugh flow-through diffusion cells. Receptor phase samples were collected over the 24-hour study period and HP content assessed using liquid chromatography–mass spectrometry analysis. Halobetasol propionate lotion 0.01% demonstrated faster tissue permeation, with receptor phase levels of 0.91% of the applied dose at 24 hours compared to 0.28% of the applied dose with HP cream 0.05%. Although there was little differentiation of cumulative receptor fluid levels of HP at 6 hours, there was significant differentiation at 12 hours. Levels of HP were lowest in the receptor phase and highest in the epidermal layers of the skin, indicating limited permeation through the epidermis to the dermis. The mean (SD) for epidermal deposition of HP following the 24-hour duration of exposure was 6.17% (2.07%) and 1.72% (0.76%) for the 0.01% lotion and 0.05% cream, respectively (Figure 1)(unpublished data, Bausch Health, 2018).
Efficacy and Safety of HP Lotion 0.01% in Moderate to Severe Plaque Psoriasis
Two articles have been published on the use of HP lotion 0.01% in moderate to severe psoriasis: 2 pivotal studies comparing once-daily application with vehicle lotion over 8 weeks (N=430),20 and a comparative “label-restricted” 2-week study with HP lotion 0.01% and HP cream 0.05% (N=150).21
HP Lotion 0.01% Compared to Vehicle
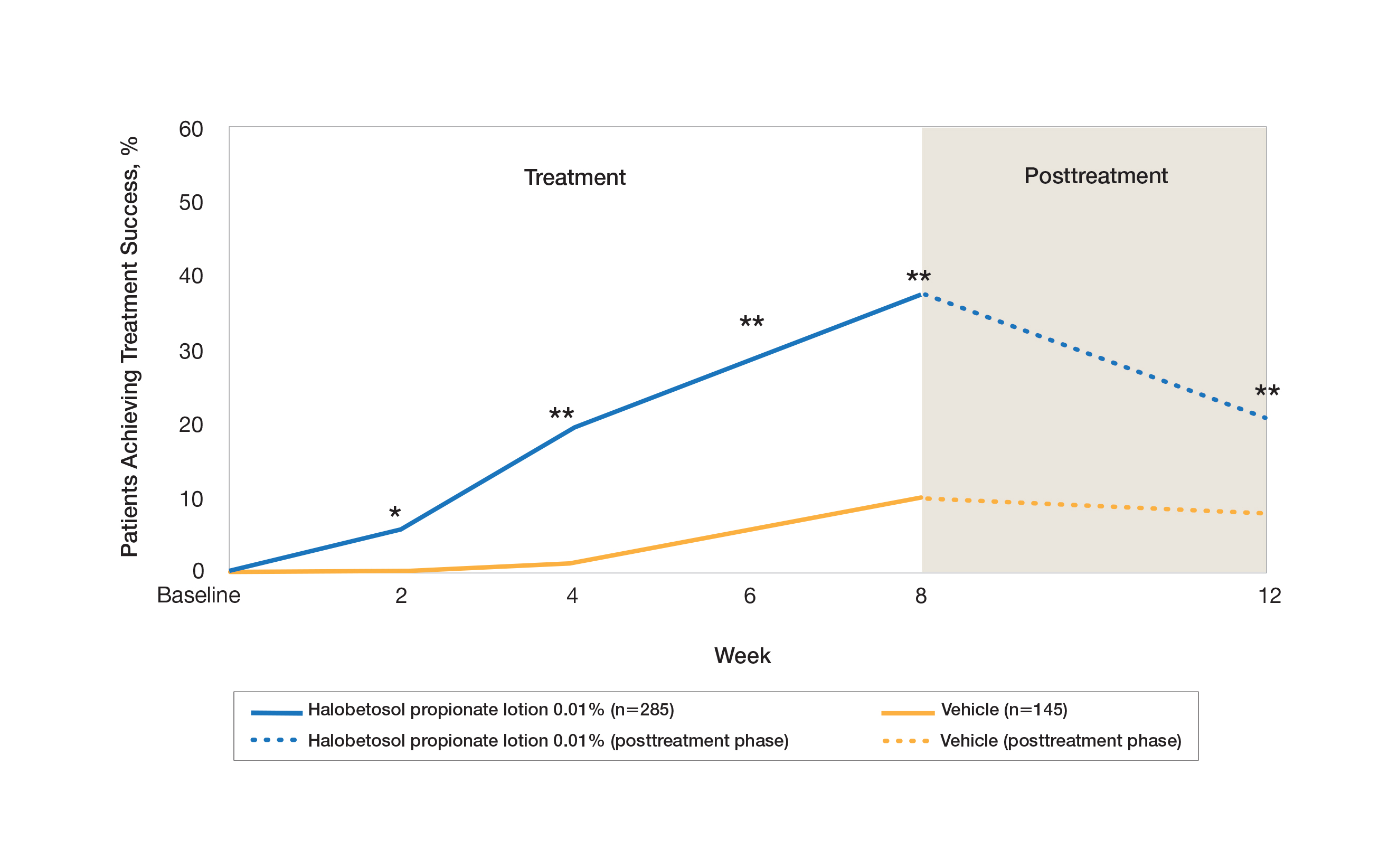
Two multicenter, randomized, double-blind, vehicle-controlled phase 3 studies investigated the safety and efficacy of once-daily HP lotion 0.01% in moderate to severe plaque psoriasis (N=430).20 Patients were treated with HP lotion 0.01% or vehicle (randomized in a 2:1 ratio) for 8 weeks, with a 4-week posttreatment follow-up. Treatment success (defined as at least a 2-grade improvement in baseline IGA score and a score equating to clear or almost clear) was significantly greater with HP lotion 0.01% at all assessment points (Figure 2)(P=.003 for week 2; P<.001 for other time points). At week 8, 37.4% of patients receiving HP lotion 0.01% were treatment successes compared to 10.0% of patients receiving vehicle (P<.001). Additionally, a 2-grade improvement from baseline for each psoriasis sign—erythema, plaque elevation, and scaling—was achieved by 42.2% of patients receiving HP lotion 0.01% at week 8 compared to 11.4% of patients receiving vehicle (P<.001). Good efficacy was maintained posttreatment that was significant compared to vehicle (P<.001).20
There were corresponding reductions in body surface area (BSA) affected following treatment with HP lotion 0.01%.20 At baseline, the mean BSA was 6.1 (range, 3–12). By week 8, there was a 35.2% reduction in BSA compared to 5.9% with vehicle. Again, a significant reduction in BSA was maintained posttreatment compared to vehicle (P<.001).20
Halobetasol propionate lotion 0.01% was well tolerated with few treatment-related AEs.20 Most AEs were application-site reactions such as dermatitis (0.7%), infection, pruritus, and discoloration (0.4% each). Mild to moderate itching, dryness, burning, and stinging present at baseline all improved with treatment, and severity of local skin reactions was significantly lower than with vehicle at week 8 (P<.001). Quality-of-life data also highlighted the benefits of active treatment compared to vehicle for cutaneous tolerability. The Dermatology Life Quality Index (DLQI) is a 10-item patient-reported questionnaire consisting of questions concerning symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment.22 Change from baseline for DLQI (how itchy, sore, painful, stinging) was significantly greater with HP lotion 0.01% at weeks 4 and 8 (P<.001). Changes in the overall DLQI score also were significantly greater with HP lotion 0.01% at both study visits (P=.006 and P=.014 at week 4 and P=.001 and P=.004 at week 8 for study 1 and study 2, respectively).20
HP Lotion 0.01% Compared to HP Cream 0.05%
Treatment success with HP lotion 0.01% also was shown to be comparable to the higher-concentration HP cream 0.05% in patients with moderate to severe psoriasis over a 2-week “label-restricted” treatment period (Figure 3). Both products were well tolerated over the 2-week treatment period. One patient reported application-site dermatitis (1.7%) with HP lotion 0.01%.21
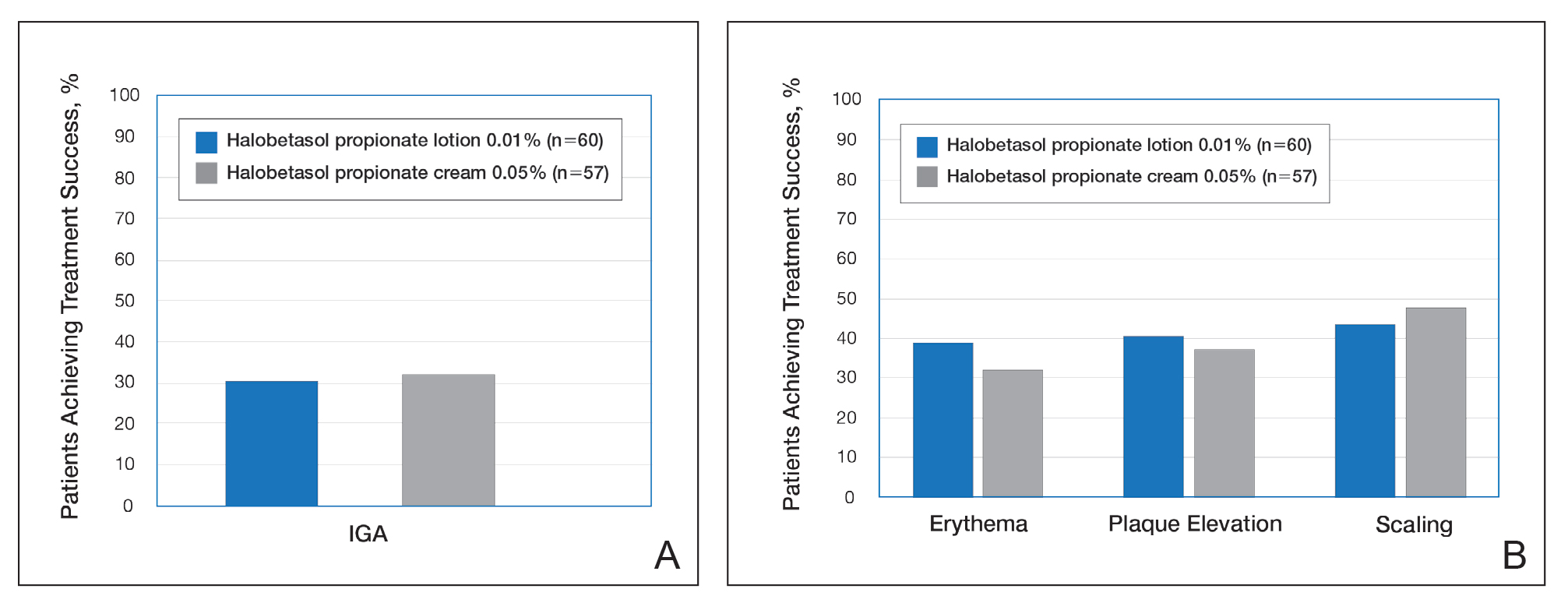
Figure 3. Treatment success following once-daily treatment with halobetasol propionate lotion 0.01% and halobetasol propionate cream 0.05% for 2 weeks.21 A, Investigator global assessment (IGA) of treatment success was defined as at least a 2-grade improvement from baseline and a score of clear or almost clear. B, Erythema, plaque elevation, and scaling treatment success was defined as at least a 2-grade improvement from baseline. All comparisons were not significantly different. Reprinted with permission from Taylor & Francis Ltd.21
Conclusion
Halobetasol propionate 0.05%—cream, ointment, and lotion—has been shown to be a highly effective short-term topical treatment for psoriasis. Longer-term treatment strategies using HP, which are important when considering management of a chronic condition, have been limited by safety concerns and labelling. However, there are data to suggest weekend or pulsed therapy may be an option.
A novel formulation of HP lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets. The polymerized honeycomb matrix and vehicle formulation form a barrier by reducing epidermal water loss and improving skin hydration. The oil droplets deliver uniform amounts of active ingredient in an optimal size for follicular penetration. Skin penetration has been shown to be quicker with greater retention in the epidermis with HP lotion 0.01% compared to HP cream 0.05%, with corresponding considerably lower penetration into the dermis.
Although there have been a number of clinical studies of HP for psoriasis, until recently there have been no comparative trials, with studies label restricted to a 2- to 4-week duration. Three clinical studies with HP lotion 0.01% have now been reported.Not only has HP lotion 0.01% been shown to be as effective as HP cream 0.05% in a 2-week comparative study (despite having one-fifth the concentration of HP), it also has been shown to be very effective and well tolerated following 8 weeks of daily use.20,21 Further studies involving longer treatment durations are required to better elucidate AEs, but HP lotion 0.01% may provide the first longer-term TCS treatment solution for moderate to severe psoriasis.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.